Research - (2021) Volume 0, Issue 0
Zinc sorption of soils of varying lithologies in a humid tropical environment
U.N. Nkwopara*Abstract
Zinc sorption capacity of soils of different parent materials in Imo state, southeastern, Nigeria was evaluated by equilibrating 2 g soil with 25 ml ZnSO4 solution containing graded zinc concentrations (0, 25, 50 and 100 mg/L) with KCl as the background electrolyte and sorbed zinc determined. Sorbed zinc was fitted to the Langmuir, Freundlich and Temkin isotherms and sorption maximum (b), affinity constant (k), bonding energy (n), distribution coefficient (Kf) and equilibrium zinc concentration (EPCo) determined. Sorption maximum was higher in shale, while the reverse was the case for the affinity constant. Zinc sorption capacity using sorption maximum followed the order coastal plain sands
Keywords
Zinc sorption, parent material, soils, Imo state, southeastern Nigeria.
Introduction
Potential toxic elements (PTE) application to the soil through composts, sewage -sludge and other waste materials increase heavy metal concentration in soils and crops. Studies have shown that the application of waste materials, including sewage-sludge, increased PTEs in the decreasing order: Zn>Cd>Ni>Cu>Pb=Hg=Cr.Zn,Cu,Ni are readily absorbed to potentially phytotoxic levels and are the principal phytotoxic elements applied to soil in sludge (Das, 2015). Zinc is an essential mineral perceived by the public today as being of exceptional biological and public health importance, significantly increasing prenatal and postnatal development. In children, it causes growth retardation, delayed sexual maturation, infection susceptibility and diarrhea (Barrow, 1993). Zinc deficiency affects about two million people in the developing world and is associated with many diseases (Prasad, 1993). Zinc deficiency has been widely reported in most semi and calcareous soils, highly weathered tropical soils and coarse-textured soils of different agro-ecological zones (Dahiya et al., 2005). It thus constitutes one of the crops most limiting nutrients in the intertropical zones (Dandanmozd et al., 2010; Perez-Novo et al., 2011). The potential for zinc sorption varies with soils of the savanna zones being higher than those of the forest zones (Banjoko et al., 1982). Two mechanisms, adsorption and precipitation, control the sorption process, with adsorption occurring at low and precipitation at high equilibrium ion concentrations. Studies of the sorption process could be undertaken using sorption isotherms, with the most frequently used being Langmuir, Freundlich and Temkin isotherms (Dandanmozd et al., 2010; Reyhanitabar et al., 2007).
Soil properties, especially pH, clay content, organic matter (OM), iron and aluminum oxides and CEC influence zinc sorption capacity, with the nature of the relationship, often estimated using correlation analysis (Azeez et al., 2018). High sorptivity of the soils has been ascribed to the nature of the dominant clay minerals, particularly kaolinite, goethite, lepidocrocite, gypsite and sesquioxides, known to have a high surface area and sorption capacity (Adetunji and Adepelu, 1987).
Civilization, Urbanization and advancement in technology have led to the accumulation of heavy metals in the environment. Unlike in the western world, tropical Africa is yet to effectively manage the side effects of this technological advancement (Azeez et al. 2018). In principle, the metal retention capacity of soil could be used as a tool in the design of remediation techniques that utilize the soil as a natural sorbent for contaminants in free water bodies (Abdu and Mohammed, 2016).
Few studies on zinc sorption on Nigerian soils have been reported (Banjoko et al., 1982; Chukwuma et al., 2010; Abdu and Mohammed, 2016; Azeez et al., 2018) contain information on soils of these parent materials in the forest zone. Therefore, this study evaluates the zinc sorption characteristics of three tropical soils of contrasting parent material and properties under mono-metal solution.
Materials and Methods
Site description and sample collection
The soil samples used were collected from three different locations: Ihiagwa, which lies between latitude 5°21' and 5°27' N and longitude 7°20' and 7°15' E, soils derived from coastal plain sands. Egbema lies between latitude 5°31' and 5°58' N and longitude 6°50' and 6°59' E, soil derived from alluvium and Amuro with soils derived from shale and lies between 5°48' and 5°53' N and longitude 7°20' and 7°25' E. The bulked soil samples used in this study were collected from a soil depth of 0-30 cm at random. The soil samples were air-dried, crushed, and sieved using a 2 mm sieve, and fine earth fractions were used for soil analysis.
Laboratory analyses
The particle size distribution was determined using the hydrometer method described by Gee and Or (2002). Bulk density was determined by the core method according to the procedure of Grossman and Reinsch (2002). It was calculated as follows;
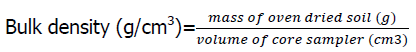 (1)
(1)
Where C=solution Zn concentration (MgL-1)
X=sorbed Zn (MgKg-1)
b=Zn sorption maximum (MgKg-1)
K=a constant related to the bonding energy (L Mg-1)
Freundlich equation: X=KfC1/n
Where, Kf=energy of adsorption or distribution coefficient
n=intensity or energy of bonding
Temkin equation: EPCO=SO/Kd
Where, So=initial or native sorbed Zn (MgKg-1)
Kd=a linear adsorption coefficient (L Mg-1)
In order to fit the soils data into Langmuir, Freundlich and Temkin isotherm, the values of equilibrium concentration (C ) sorbed were transformed into C/X (Langmuir) and log C and log X (Freundlich).
Statistical analysis
The data collected were analyzed using correlation. The simple correlation was performed using Genstat statistical package (Buysse et al., 2004).
Results and Discussion
Table 1 showed that clay content ranged from 107.6–411.0 g/kg; the highest clay value (411.0 g/kg) was recorded in shale clay soil and the least in alluvial soil. The shale clay soil (Amuro) had the highest amount of clay, indicating the low potential for leaching of pollutants such as zinc (Nyles and Ray, 1999). This implies that shale clay soil can be less polluted in the case of zinc pollution. The pH of the soils ranged from 5.34–5.88; the highest pH value (5.88) was recorded in alluvial soil and the most negligible value (5.34) in shale clay soil. The highest organic carbon and total nitrogen were recorded in alluvial soil, while the highest available phosphorus was observed in shale clay soil. Shale clay soil had the highest exchangeable calcium, magnesium, potassium but the least exchangeable sodium. The Effective cation exchange capacity (ECEC) is in the order shale clay>alluvial soil>coastal plain sands. The pH is a fundamental property that significantly affects soil contaminants' solute concentration and sorption (Ogunmethin et al., 2005; Azeez et al., 2018). The higher organic carbon in alluvial soil implies that organic matter, an index of organic carbon, can hold or retain the metals, and therefore, it may increase the sorption ability of the soil. The low concentration of exchangeable bases in these soils may be the reason for the low ECEC recorded in these soils 4.53 cmol/kg, 1.52 cmol/kg, and 0.81cmol/kg for shale clay, alluvial soil and coastal plain sands, respectively.
| Soil properties | Shale clay | Alluvium | Coastal plain sands |
|---|---|---|---|
| Sand (g/kg) | 366 | 792.5 | 806 |
| Silt (g/kg) | 223.2 | 99.9 | 83.2 |
| Clay(g/kg) | 411 | 107.6 | 111 |
| BD(g/cm3) | 1.2 | 1.25 | 1.37 |
| MC (%) | 14.81 | 11.69 | 12.85 |
| TP (%) | 52.8 | 51.3 | 45 |
| pH (H2O) | 5.34 | 5.88 | 5.87 |
| OC (g/kg) | 2.92 | 3.86 | 2.72 |
| TN (g/kg) | 0,24 | 0.38 | 0.29 |
| AV. P (mg/kg) | 8.75 | 8.38 | 5.74 |
| Ca (cmol/kg) | 1.088 | 0.72 | 0.667 |
| Mg (cmol/kg) | 0.688 | 0.09 | 0.12 |
| K (cmol/kg) | 0.025 | 0.02 | 0.02 |
| Na (cmol/kg) | 0.016 | 0.018 | 0.022 |
| Al (cmol/kg) | 3.1 | 1.35 | - |
| H (cmol/kg) | 1.6 | 0.67 | - |
| TEA (cmol/kg) | 4,70 | 2.02 | - |
| ECEC (cmol/kg) | 4.53 | 1.52 | 0.812 |
| TEB (cmol/kg) | 1.74 | 0.85 | 0.812 |
| TZn (mg/kg) | 0.741 | 0.745 | 0.449 |
exchangeable bases, TZn=Total zinc (Okoli et al., 2017).
Table 1. Selected physicochemical properties of soils of varying parent materials.
Sorption characteristics of zinc
The sorption characteristics of zinc on the studied soils are presented in Table 2. The result shows that as the amount of zinc added increased, the quantity sorbed (C ) and the equilibrium concentration (X) also increased for the soils of the three-parent materials. The high concentration in equilibrium with increased zinc application could be due to the satisfaction of the soil sorption complex and thus the release of a greater quantity of zinc into the soil solution. The amount of zinc sorbed was higher in alluvial soil (Egbema) than in the other soils. This could be due to the higher value in the pH and organic carbon of this soil. Higher pH leads to a higher net negative surface charge and thus increases soil affinity for metal ions, while higher organic carbon means a higher ability to retain metal ions. This result agrees with Nkwopara et al. (2012) and Abdu and Mohammed (2016) on adsorption of lead on some variable charge soils in china and adsorption of Pb, Cd and Zn in savanna soil, respectively. C/X decreased with the added zinc except shale clay (Amuro) for each of the sites, which increased. Also, as log C increased, log X equally increased.
| Parent materials | Added Zn | C | X | C/X | Log C | Log X |
|---|---|---|---|---|---|---|
| Shale clay | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 5.47 | 20 | 0.3 | 0.74 | 1.3 | |
| 50 | 11.67 | 38 | 0.3 | 1.07 | 1.58 | |
| 100 | 28.97 | 71 | 0.4 | 1.46 | 1.96 | |
| Alluvium | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 7.47 | 18 | 0.4 | 0.87 | 1.26 | |
| 50 | 6.87 | 43 | 0.2 | 0.84 | 1.63 | |
| 100 | 7.97 | 92 | 0.1 | 0.9 | 1.96 | |
| Coastal plain sands | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 9.7 | 15 | 0.6 | 0.99 | 1.2 | |
| 50 | 9.8 | 40 | 0.2 | 0.99 | 1.6 | |
| 100 | 9 | 91 | 0.1 | 0.95 | 1.96 |
Table 2. Zinc sorption data on soils of varying parent materials.
Sorption studies
Table 3 shows the sorption parameters obtained from the various isotherms (Langmuir, Freundlich and Temkin). From the Langmuir isotherm, the sorption maximum (b), defined as the amount of sorbate that a sorbent can sorb, was higher in shale clay (Amuro) (91 mg kg-1) than the other soils. This implies that the capacity for zinc sorption will be higher in shale clay than in the other soils. The tenacity (k) with which sorbed zinc was held was in the following order alluvial soil>coastal plain sands soil>shale clay soils. This shows that though the capacity to hold zinc was higher in shale clay soil, its tenacity was very low, indicating that zinc will be more available in shale clay soil than the other soils. The higher tenacity of alluvial soil may result from higher organic matter of the soil than the other soils. Maximum buffering capacity (MBC) described as the resistance to changes in soil solution ion concentration (Uzoho et al., 2014) was lower in shale clay soil than the other soils. The soil MBC seriously influences the availability of zinc. The energy of bonding (n) which measures the intensity of adsorption, was more significant in shale clay soil (0.77 mL g-1) and least in alluvial soil (0.53 mLg-1). The value of n not between 1 -10 confirms the absorbent's poor adsorption potential (Geethakarthi and Phanikumar, 2011). The values of n for shale clay soil, alluvial soil and coastal plain sands soil were 0.77, 0.54 and 0.62, respectively. The capacity of adsorption (Kf) (was greater in shale clay (1.34 mLg-1) and least in alluvial (1.02 mLg-1). The Kf was greater in shale clay than the other soils, indicating that shale clay soil has a greater adsorptive capacity for zinc than the other soils. EPCO determines the sorbate concentration in equilibrium solution concentration at which the amount sorbed is equal to that desorbed or soil zinc availability (Litaor et al., 2005; Brand-klibanski et al., 2007). EPCO was greater in shale clay soil than in the other soils. The soil EPCO increased with an increase in So or native zinc and decreased in the distribution coefficient or native sorbed zinc (Kd). This implies that in soils with low zinc sorption, the native zinc and EPCO will be high, thus increasing zinc solubility and mobility and the tendency for the pollution of the environment. This result aligns with Uzoho et al. (2014) on the phosphorus sorption capacity of soils of different land-use types in Mbaise, Southeastern Nigeria. The highest EPCO of shale clay soil occurred at values equivalent to lowest K, MBC and Kd. The high value of b in shale clay soil compared to other soils indicate that more zinc was sorbed on the soil than the other soils, while the high value of k in alluvial soil compared to the other soils shows that zinc was held more tenaciously on the soil than the other soils. The high value of EPCO in shale clay soils compared to other soils indicates that zinc solubility and mobility were higher on the soil than the other soils.
| Langmuir | isotherm | Freundlich | Temkin | isotherm | ||||
|---|---|---|---|---|---|---|---|---|
| Parent materials | b (mg kg-1) | K (Lmg-1) | MBC (Mgkg-1) |
Kf (mlg-1) | n (mlg-1) |
So (Lmg-1) | Kd {mg g-1) |
EPCo (Lmg-1) |
| Shale clay | 91 | 0.10 | 9.1 | 1.34 | 0.77 | 4.94 | 2.369 | 2.09 |
| Alluvium | 33.33 | 3.33 | 110.9 | 1.02 | 0.53 | 2.80 | 7.36 | 0.38 |
| Coastal plain sands |
30.3 | 2.75 | 83.3 | 1.04 | 0.62 | 3.40 | 4.65 | 0.73 |
Table 3. Zinc sorption parameters of the soil of the varying parent materials.
Relationship between sorption parameters and selected soil properties
Table 4 depicts the relationship between sorption parameters and selected soil properties. The parameters b, k, n, Kf, EPCO and Kd were significantly correlated with Effective cation exchange capacity (ECEC), phosphorus, clay content and sand content. Other researchers have also reported a correlation between n and clay and ECEC (Dandanmozd et al., 2010; Reyhanitabar et al., 2007). Sorption maximum (b) had a positive significant relationship with ECEC (r=0.961), P (r=0.6295), Ca (r=0.9949)( p ≤ 0.05). Sorption maximum (b) and Adsorption capacity (Kf) had a negative significant relationship with %BS (r=- 0.9407 and r=-0.9699, p ≤ 0.05) respectively. Adsorption capacity (Kf) had a positive significant relationship with P, ECEC, clay content and Ca (r=0.5486, 0.9287, 0.9989, 0.9799, p ≤ 0.05) respectively. The tenacity (k) had a positive significant relationship with % BS and sand content (r=0.9912 and 0.9809, p ≤ 0.05) respectively, while it had a negative significant relationship with P, ECEC, clay content and Ca (-0.4504, -0.8808, -0.9873, -0.9511, p ≤ 0.05) respectively. The energy of bonding (n) had a significant negative relationship with %BS and sand content (r=-0.9969 and 0.9182, p ≤ 0.05) respectively, while it had a significant positive relationship with ECEC, clay content and Ca (0.7620, 0.9321, 0.8652, p ≤ 0.05) respectively. The significant positive relationship between ECEC and clay content and b and Kf show that the adsorption capacity of the soils for zinc increased with an increase in ECEC and clay content.
| Soil properties | b | k | MBC | n | Kf | So | Kd | EPCo |
|---|---|---|---|---|---|---|---|---|
| Sand | -0.9998 | 0.9808 | 0.9576 | -0.9182 | -0.9966 | -0.8252 | -0.9547 | -0.9755 |
| Clay | 0.9985 | -0.9873 | -0.9675 | 0.9321 | 0.9989 | -0.8454 | 0.9650 | 0.9839 |
| P | 0.6295 | -0.4504 | -0.3628 | 0.2535 | 0.5486 | -0.0632 | 0.3535 | 0.4274 |
| Ca | 0.9949 | -0.9511 | -0.9171 | 0.8652 | 0,9799 | -0.7530 | 0.9130 | 0.9428 |
| ECEC | 0.9611 | -0.8808 | -0.8313 | 0.7620 | 0.9287 | -0.6236 | 0.8257 | 0.8683 |
Table 4. Correlation between soil properties and sorption parameters.
Conclusion
From these studies, more zinc is sorbed on shale clay soils and is more soluble and mobile, while zinc is held tenaciously on alluvial soils than the other soils. The shale clay soil has the potential of adsorbing more zinc from polluted solutions, while the alluvial soil can hold zinc more tightly than the other soils. The adsorption capacity of the zinc increased with an increase in ECEC and clay content. Zinc sorption capacities of these soils varied with the parent material, with the capacity higher in shale clay soil than others.
References
Abdu, N., Mohammed, I. (2016). Adsorption-solubility equilibria and speciation of Pb, Cd, and Zn in a savanna soil. Spanish Journal of Soil Science, 6:244-260.
Adetunji, M.T., and Adepetu, J.A., (1987). Sulphur sorption characteristics of soils of Southwestern, Nigeria, Nigerian Journal of Soil Science, 7:63-72.
Oladipupo Azeez, J., Olabisi Hassan, A., Blessing Olowoboko, T. (2018). Differential sorption behavior of cadmium, lead, zinc, and copper in some tropical soils and their environmental implications. Communications in Soil Science and Plant Analysis, 49:1707- 1718.
Banjoko, V.A., Solubo, R.A., (1982). Zinc adsorption by typical Nigerian Soils. Nigerian Journal of Soil Science, 3:28-43.
Barrow, N.J. (1993). Mechanisms of reaction of zinc with soil and soil components. In zinc in soils and plants. Springer, Dordrecht, pp:15-31.
Brady, N.C., Weil, R.R., Weil, R.R. (2008). The nature and properties of soils. Upper Saddle River, NJ: Prentice Hall, 13:662-710. Brand-Klibanski, S., Litaor, M.I., Shenker, M. (2007). Overestimation of phosphorus adsorption capacity in reduced soils: An artifact of typical batch adsorption experiments. Soil Science Society of America Journal, 71:1128-1136.
Buysse, W., Stern, R., Coe, R. (2004). GenStat discovery edition for everyday use. ICRAF Nairobi, Kenya.
Chukwuma, M.C., Eshett, E.T., Onweremadu, E.U., Okon, M.A. (2010). Zinc availability in relation to selected soil properties in a crude oil polluted eutric tropofluvent. International Journal of Environmental Science & Technology, 7:261-270.
Dahiya, S., Shanwal, A.V., Hegde, A.G. (2005). Studies on the sorption and desorption characteristics of Zn (II) on the surface soils of nuclear power plant sites in India using a radiotracer technique. Chemosphere, 60:1253-1261.
Dandanmozd, F., Hosseinpur, A.R. (2010). Thermodynamic parameters of zinc sorption in some calcareous soils. Journal of American Science, 6:298-304.
Das, D.R. (2015). Introductory Soil Science. Kalyani Publisher, B-1/1292 Rajinder, Nager, Ludhiana, India Ed, 4:879. Gee, G.W., Or, D. (2002). Particle-size analysis. Methods of Soil Analysis. 4:255-293.
Geethakarthi, A., Phanikumar, B.R. (2011). Adsorption of reactive dyes from aqueous solutions by tannery sludge developed activated carbon: Kinetic and equilibrium studies. International Journal of Environmental Science Technology, 8:561-570.
Grossman, H.B., Reinsch, T.G., (2002). Bulk density and linear extensibility in: Dane, J.H., Topp, G.C. (Eds), Part 4. Physical Method, pp:201-225.
Hendershot, W.H., Lalande, H., Duquette, M. (1993). Soil reaction and exchangeable acidity. Soil Sampling and Methods of Analysis. Jackson, M.L. (1962). Soil chemical analysis. Prentice Hall Inc. New York, pp:498.
Jackson, M.L. (1964). Soil chemical analysis. Prentice Hall Inc. New York, Englewood Cliffs, pp:86-92.
Litaor, M.I., Reichmann, O., Haim, A., Auerswald, K., Shenker, M. (2005). Sorption characteristics of phosphorus in peat soils of a semiarid altered wetland. Soil Science Society of America Journal, 69:1658-1665.
Nelson, D.W., Sommers, L.E. (1996). Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 3 Chemical Methods, 5:961-1010.
Ugochukwu, N., Ali, I., Fu, Q., Zhu, J., Jiang, G., Hu, H. (2012). Sorption of lead on variable-charge soils in China as affected by initial metal concentration, pH and soil properties. Journal of Food Agriculture & Environment, 10:1014-1019.
Nyle, C.B., Ray, R.W. (1999). The nature and properties of soils. Pearson education, New Jersey, p:881.
Oguntimehin, I.I., Ipinmoroti, K.O., Aiyesanmi, A.I. (2005). Evaluation of heavy metals in soils at automobile workshop in Akure. Nigerian Journal of Soil Science, 15:151-153.
Okoli, N.H., Uzoho, B.U., Onweremadu, E.U., Ahukaemere, C.M., Osisi, A.F., Aliba, E.O. (2017). Status of available micronutrients in soil profiles of different parent materials in Imo State, Southeastern, Nigeria. Nigerian Journal of Soil Science, 27:40-52.
Olsen, S.R., Sommers, L.E. (1982). In Page AL et al. (ed) Methods of soil analysis, part 2. American Social Agron Inc, Madison, WI, 2:403-430.
Pérez-Novo, C., Bermúdez-Couso, A., López-Periago, E., Fernández-Calviño, D., Arias-Estévez, M. (2011). Zinc adsorption in acid soils: influence of phosphate. Geoderma, 162:358-364.
Prasad, A.S. (1993). Essentiality and toxicity of zinc. Scandinavian Journal of Work, Environment & Health, pp:134-136. Reyhanitabar, A., Karimian, N., Ardalan, M., Savaghebi, G., Ghannadha, M. (2007). Comparison of five adsorption isotherms for prediction of zinc retention in calcareous soils and the relationship of their coefficients with soil characteristics. Communications in Soil Science and Plant Analysis, 38:147-158.
Uzoho, B.U., Ihem, E., OnwudikeS, A.L., Nkwopara, U., Opara, I.U., Okon, M., Orji, J.C. (2014). Phosphorus sorption capacity of soils in relation to land use types in Mbaise, Southeastern, Nigeria. Journal of Chemical, Biological and Physical Sciences, 4:1710- 1720.
Author Info
U.N. Nkwopara*Citation: Nkwopara U.N. (2021). Zinc sorption of soils of varying lithologies in a humid tropical environment. Ukrainian Journal of Ecology, 11 (6), 1-6.
Received: 30-Jun-2021 Accepted: 10-Jul-2021 Published: 23-Aug-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
