Research - (2021) Volume 0, Issue 0
Validation of the quantitative determination method of spectinomycin in muscle samples by the immunoenzyme analysis method
K.S. Myagka1, S.A. Tkachuk2, I.V. Yatsenko3, K.O. Rodionova4*, M.V. Kostiuk1, N.V. Liniychuk1, V.P. Lyasota5, V.M. Zhylina3, I.L. Tsivirko3 and L.B. Savchuk6Abstract
Antimicrobial residues in raw materials and products of animal origin are regulated by the following EU regulations: Commission Regulation (EC) No 37/2010, Council Directive No 96/23/EEC, Council Regulation (EC) 2377/90, Codex Alimentarius Commission. For spectinomycin in animal muscles, the European Union has set Maximum Allowed Levels (MRLs): 300 μg/kg, according to the Codex Alimentarius Commission-500 μg/kg. A wide variety of spectinomycin methods are used worldwide, including Fluorescence Latex Immunoassay (FLI), Micellar Electrokinetic Capillary Chromatography In Combination With Ultraviolet Detection (MEKC-UVD), Enzyme-Linked Immunosorbent Assay (HPA), high-efficiency electrocardiography, and high-performance liquid chromatography with Fluorescence Detection (FLD) of microbiological inhibition. The purpose of the study was to validate the ELISA method and the liquid chromatography method with the mass spectrometric detector (LC/MS/MS) to determine the residual spectinomycin content in muscle samples (cattle, pigs, chickens, geese, and turkeys). The determination of the spectinomycin residue was performed by ELISA on a Tecan Sunrise spectrophotometer (manufactured by Sunrise, Austria) using a test system for competitive enzyme-linked immunosorbent assay Kwinbon Biotech Spectinomycin (Cat. No.: KA02701H) and liquid chromatography. -spectrometric detector on the device Waters Xevo (USA). The chromatograph was equipped with an ACQUITY UPLC BEH C18 analytical column, a two-quadrupole mass spectrometric detector, a positive ionization spray, and MasLynx results calculation software. Validation characteristics have been established for the detection of spectinomycin residues in muscle samples, such as: detection ability (CCβ) is 40.0 μg/kg, cut-off level is 32.9 μg/kg. The lowest spectinomycin content that can be detected by ELISA using a test system for the competitive enzyme-linked immunosorbent assay Kwinbon Biotech (China) is 3.0 μg/kg. The main validation characteristics were also determined by liquid chromatography with a mass spectrometric detector. CCα is 358.08 and CCβ-459.52.
Keywords
Validation characteristics, muscles, ELISA, LC/MS/MS, Spectinomycin.
Introduction
Spectinomycin is an aminoglycoside antibiotic that inhibits bacterial protein synthesis and acts on the 30S ribosome subunit, and its antibacterial mechanism mainly prevents the binding of ribonucleic acid to ribosomes, which inhibits protein synthesis and leads to bactericidal action (Butler et al., 2018). Spectinomycin has strong antibacterial activity against gram-negative bacteria and weak activity against gram-positive bacteria, so spectinomycin is usually used in combination with lincomycin, which does not affect gram-negative bacteria, but has a strong antibacterial effect on gram-positive bacteria. In combination, these antibiotics are used to treat infections caused by gram-positive and gram-negative bacteria and to treat diarrhea in piglets and chronic respiratory infections in chickens caused by Mycoplasma hyopneumoniae and Mycoplasma pneumoniae (Verrette et al., 2019).
However, spectinomycin can damage the eighth cranial nerve, have a toxic effect on the kidneys, and block neuromuscular transmission; lincomycin has severe side effects, damages the gastrointestinal tract and liver, and even causes anaphylactic shock and death (Wang et al., 2020).
In general, antibiotics are used in animal husbandry for three main purposes: as therapeutic agents for the treatment of established infections, as prophylactics to prevent the development of bacterial diseases in clinically healthy animals, and as growth stimulants in feed. Despite the adoption in many countries of regulations prohibiting the use of antibiotics for disease prevention and growth promotion, feed containing antibiotics can still be purchased without veterinary prescriptions in a number of major animal-producing countries, including the United States, Canada, China, and Australia (Maron et al., 2013).
In October 2019, the European Veterinary Surveillance for Antimicrobials (ESVAC) published a report in which antibiotic sales of antibiotics for use in animals in Europe fell by more than 32% between 2011 and 2017. Polymyxin sales decreased by 66% and sales of third- and fourth generation decreased by more than 20%. These classes include colistin and other antibiotics used to treat serious infections in humans caused by bacteria resistant to most treatments. However, this report notes that the situation in Europe remains a contrast. In 19 of the 25 countries that provided data for 2011-2017, antibiotic sales declined by more than 5%, but 3 countries recorded an increase of more than 5% (European Medicines Agency, 2019).
Irrational use of veterinary drugs-the introduction of insufficient (subtherapeutic) doses, increasing the intervals between drug administration, unreasonable prolongation of treatment, violation of zoohygienic and sanitary norms not only reduces the therapeutic efficacy of antimicrobial drugs, but also contributes to resistant microorganisms, pathogens (Lisicyn, 2015).
Antimicrobial residues in raw materials and products of animal origin are regulated by the following EU regulations: Council Directive 96/23/EC, 1996; Council Regulation (EC) N 2377/90, 1990; Commission Regulation (EU) No 37/2010, 2009; Codex Alimentarius, 2018.
For spectinomycin in animal muscles, the European Union has set maximum allowed levels (MRLs): 300 μg/kg, according to the Codex Alimentarius Commission-500 μg/kg.
There are many different methods of spectinomycin use in the world, including Fluorescence Latex Immunoassay (FLI), Micellar Electrokinetic Capillary Chromatography In Combination With Ultraviolet Detection (MEKC-UVD), Enzyme-Linked Immunosorbent Assay (HPA), high-efficiency electrocardiography), high-performance liquid chromatography with Fluorescence Detection (FLD), microbiological inhibition (Wua et al., 2019; Wang et al., 2020), and others.
Thus, by liquid chromatography followed by mass spectrometric detection, the concentration was selected followed by purification by weak cation-exchange solid phase extraction. The precision of the method for muscle tissue varied from 83 to 128%, during the day from 2.2 to 17.3% (RSDr, n=7, MRL level). According to the results, it was concluded that this method can control levels of 50 to 10,000 μg/kg-1 for muscle tissue (Holthoon et al., 2008).
At the same time, a simple, fast and sensitive method for the determination and confirmation of aminoglycoside residues was performed using extraction with trichloroacetic acid solution and purification by liquid chromatography with tandem mass spectrometry. Calibration matrix curves in the linear range around the MPC, as well as the internal standard of tobramycin, were used for quantification. Calculated test parameters such as CCα, CCβ, recovery (94-103%), relative repeatability RSDr (3.6-9.7%), and relative reproducibility within the laboratory RSD wR (4.6-10.0%) met the requirements of the Commission Decision 2002/657/EC (Bohm et al., 2013; Arsand et al., 2016; Wang et al., 2018).
In addition, high performance chromatography with tandem mass spectrometry is enhanced by adding sodium acetate in methanol (5 mg L-1 at a flow rate of 0.2 ml min-1). Limits for the quantification of aminoglycosides ranged from 0.19 to 2.5 ng ml-1 (Asakawa et al., 2018).
Instead, gas chromatography and tandem mass spectrometry quantified spectinomycin residues. To do this, the samples were extracted and separated by accelerated solvent extraction, and then purified and enriched in solid phase extraction. The residues obtained were derivatized with trifluoroacetamide for 60 min at 75 C, and the derived products were introduced into the GC-MS/MS system. Under optimized conditions, the Limits Of Detection (LOD) and the Limits Of Quantification (LOQ) of spectinomycin in the samples were 2.5-4.6 μg/kg, the cutoff level was 79.7%-94.2%, and the coefficients of determination were 0.9992-0.9998 and Relative Standard Deviations (RSD)-1.2% -3.5% (Guoa et al., 2021).
Currently, the determination of antimicrobial residues in Ukraine remains a problematic issue. Enzyme-Linked Immunosorbent Assay (ELISA) differs favorably among other screening methods by high sensitivity, specificity, simplicity and speed of execution, availability and stability of reagents, the ability to computer processing of measurement results, and automation of stages of analysis, which provides high test efficiency (Yanovych et al., 2014; Myagka et al., 2018).
The aim of the work is to validate the ELISA method and the liquid chromatography method with a mass spectrometric detector (LC/MS/MS) to determine the residual spectinomycin content in muscle samples (cattle, pigs, chickens, geese and turkeys).
Materials and Methods
The research was carried out on the basis of the State Research Institute for Laboratory Diagnostics and Veterinary Sanitary Examination. The study material was a spectinomycin solution of spectinomycin with a concentration of 1 mg/l, 40 control muscle samples that did not contain the target analyte, and 40 muscle samples enriched with a standard solution of spectinomycin to the target concentration (CCC)-40.0 μg/kg.
The Sigma Aldrich spectinomycin standard, 40 muscle samples without analyte, and 120 muscle samples with analyte addition at concentrations of 150, 300 and 600 μg/kg were used for the LC/MS/MS method. Determination of the spectinomycin residue was performed by ELISA on a Tecan Sunrise spectrophotometer (manufactured by Sunrise, Austria) using the Kwinbon Biotech Competitive Enzyme Assay (Cat. No.: KA02701H) test system (Commission Decision 2002/657/EC, 2002) and liquid chromatography with a mass spectrometric detector on a Waters Xevo instrument (USA). The chromatograph was equipped with an ACQUITY UPLC BEH C18 analytical column, a two-quadrupole mass spectrometric detector, a positive ionization spray, and MasLynx results calculation software.
Results and Discussion
The distribution of control and spectinomycin-enriched muscle samples (cattle, pigs, chickens, geese, and turkeys) is shown in Table 1.
| Samples | 1 day | 2 day | 3 day | 4 day | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cattle muscles | 5 | Cattle muscles | 5 | Cattle muscles | 5 | Cattle muscles | 5 | |||
| 5 | 5 | 5 | 5 | |||||||
| Control | Turkey muscles | 2 | Pig muscles | 2 | Chicken muscles | 2 | Pig muscles | 2 | ||
| Enriched | 2 | 2 | 2 | 2 | ||||||
| Control | Pig muscles | 1 | Chicken muscles | 1 | Turkey muscles | 2 | Chicken muscles | 2 | ||
| Enriched | 1 | 1 | 2 | 2 | ||||||
| Control | Goose muscles | 1 | Turkey muscles | 1 | Goose muscles | 2 | Goose muscles | 2 | ||
| Enriched | 1 | 1 | 2 | 2 | ||||||
Table 1. Distribution of muscle samples.
To obtain an analyte concentration of 40.0 μg/kg, the antibiotic standard additive was calculated as follows: determine the amount of analyte to be added to the 100 g sample using the proportion:
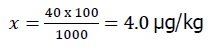
Where, X-μg of analyte per 100 g of sample.
40.0 μg of analyte per 1000 g of sample;
It was further determined: In what volume of the diluted standard is a certain amount of analyze, using the proportion:
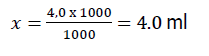
where, 1000 μg of analyte in 1000 ml;
4.0 μg of analyte in X ml.
Thus, for application per 100 g of control sample, it is necessary to take 4 ml of a standard spectinomycin solution with a concentration of 1000 μg/l.
Samples with added additive should be thoroughly mixed and examined according to the guidelines (Miahka, 2018). The results of the study are shown in Table 2.
| Sample No. | Control samples | Enriched samples at the level of CKS 40.0 µg/kg |
|---|---|---|
| 1 | 0.7 | 42.7 |
| 2 | 1.0 | 35.0 |
| 3 | 0.7 | 32.9 |
| 4 | 0.9 | 40.0 |
| 5 | 1.0 | 42.3 |
| 6 | 0.6 | 37.3 |
| 7 | 0.5 | 38.4 |
| 8 | 0.4 | 44.3 |
| 9 | 0.6 | 37.2 |
| 10 | 0.6 | 34.7 |
| 11 | 1.0 | 42.2 |
| 12 | 0.9 | 37.0 |
| 13 | 0.9 | 38.4 |
| 14 | 1.1 | 39.1 |
| 15 | 1.5 | 34.2 |
| 16 | 0.7 | 43.6 |
| 17 | 0.9 | 38.7 |
| 18 | 1.0 | 34.1 |
| 19 | 1.1 | 37.9 |
| 20 | 1.7 | 41.3 |
| average | 0.9 | 38.6 |
| SD | 0.3 | 3.4 |
| RSD, % | 8.8 | |
| Recovery, % | 96 |
Table 2. Results of the analysis of control and spectinomycin-enriched samples (cattle muscles), µg/kg.
According to the data in Table 2, the highest value (highest response) for control samples is 1.5 μg/kg and the lowest value for enriched samples (lowest response)-32.9 μg/kg. According to the results obtained, none of the responses for enriched samples coincides with the range of responses for control samples. Therefore, the detection ability (CCβ) by this screening method is less than or equal to 40.0 μg/kg.
The cutoff level of this test-32.9 μg/kg means that any result higher than this level can be considered screening positive and therefore exceeds the detection ability (CCβ) (β-error <5%).
Due to the fact that the normative limit (CCβ) was established for cattle muscles, and the method will be applied to other muscle types, the detection ability to detect must be determined by examining control samples from other animal species (pigs, chickens, geese, turkeys). and enriched at the level of the target screening concentration used for the primary matrix (40.0 μg/kg). The results of the study are shown in Table 3.
| Sample No. | Type of matrix | Control samples | Enriched samples at the level of CKS 40.0 µg/kg |
|---|---|---|---|
| 1 | Pig muscles | 0.9 | 40.1 |
| 2 | Pig muscles | 1.2 | 39.3 |
| 3 | Pig muscles | 1.1 | 34.0 |
| 4 | Pig muscles | 2.4 | 41.2 |
| 5 | Pig muscles | 1.6 | 36.2 |
| 6 | Chicken muscles | 0.9 | 32.9 |
| 7 | Chicken muscles | 1.1 | 38.3 |
| 8 | Chicken muscles | 1.0 | 35.8 |
| 9 | Chicken muscles | 1.1 | 35.6 |
| 10 | Chicken muscles | 2.1 | 48.4 |
| 11 | Goose muscles | 1.1 | 33.2 |
| 12 | goose muscles | 1,1 | 37.2 |
| 13 | goose muscles | 0.6 | 35.0 |
| 14 | goose muscles | 0.7 | 34.9 |
| 15 | goose muscles | 1.0 | 39.6 |
| 16 | turkey muscles | 1.5 | 36.8 |
| 17 | turkey muscles | 1.1 | 36.8 |
| 18 | turkey muscles | 1.0 | 34,9 |
| 19 | turkey muscles | 1.2 | 34.9 |
| 20 | turkey muscles | 0.8 | 39.4 |
| average | 1.2 | 37.2 | |
| SD | 0.4 | 3.5 | |
| RSD, % | 9.5 | ||
| Recovery, % | 93 | ||
Table 3. Results of the analysis of control and spectinomycin-enriched samples (muscles of pigs, chickens, geese and turkeys),µg/kg.
According to the figures in Table 3, the cutoff limit for the main validated matrix (beef) is 32.9. According to the data obtained in Table 3, no sample was defined as screening negative. All enriched samples are defined above the cutoff level, so the method can be applied to the above matrices.
The validation of the methodology by the LC/CU/CU method was carried out in accordance with the Decision 2002/657/EC of the European Commission Decision 2002/657/EC of 12 August 2002, which ensures the implementation of the Directive 96/23/EC on the effectiveness of analytical methods and the interpretation of results.
During the adaptation of the method, adjustments were made to spectinomycin by introducing a standard solution of high concentration on the detector. To do this, the parent ion, cone voltage, daughter ions, and capillary voltage were determined. Identification was carried out by retention time, the presence of the corresponding ions and the ratio of their intensity (mother ion for spectinomycin-351.2, daughter ions-207.2 and 333.2).
The calculations were performed using InterVal Software of Guo Data GmbH (Germany). The study was carried out for 2 days by two operators. Parameters were determined from samples enriched in a analyte at the level of 150 μg/kg, 300 and 600 μg/kg. Preliminary samples were taken for analysis. Extraction was calculated from the concentration of control samples loaded with a certain amount of analyte. CCα and CCβ were determined by a calibration curve constructed for enrichment with different concentrations of the matrix standard.
As a result of the validation and calculations obtained CCα and CCβ, which is shown in Table 4.
| Validation parameters/Indicators | Spectinomycin |
|---|---|
| CCα | 358.08 |
| CCβ | 459.52 |
Table 4. Validation results of the study of animal muscles using the method of LC/MS/MS.
Indicators for determining repeatability, internal laboratory reproducibility, and percentage of return for different levels of concentrations are presented in Table 5.
| Concentration, µg/kg |
Sr, MKr/Kr | Rel. Sr, % | Swr, MKr/Kr | Rel. Swr, % | Return percentage, % |
|---|---|---|---|---|---|
| 150 | 33.317 | 22.2 | 33.317 | 22.2 | 97.1 |
| 225 | 32.802 | 14.6 | 32.802 | 14.6 | 98.6 |
| 300 | 32.550 | 10.9 | 34.876 | 11.6 | 99.3 |
| 450 | 32.302 | 7.2 | 44.248 | 9.8 | 100.1 |
| 600 | 32.180 | 5.4 | 53.692 | 8.9 | 100.5 |
Table 5. Repeatability, internal laboratory reproducibility, and percentage of return for spectinomycin.
The results obtained satisfy the requirements of the Commission Regulation (EC) No 37/2010, where spectinomycin in animal muscles is regulated at the level of 300 μg/kg.
Conclusion
Validation characteristics for the determination of spectinomycin residues in muscle samples have been established, such as: the ability to detect (CCβ) by this screening method is 40.0 μg/kg, the cut-off level is 32.9 μg/kg. The lowest level of spectinomycin content that can be detected by ELISA using the test system for enzyme-linked immunosorbent assay Kwinbon Biotech Spectinomycin (Cat. No.: KA02701H) (China) is 3.0 μg/kg. Validation characteristics are established for determination of residual spectinomycin content in muscle samples using the LC/MS/MS method, such as the decision limit (CCα), which is 358.08 μg/kg and CCβ-459.52 μg/kg.
References
Arsand, J.B., Jank, L., Martins, M.T., Hoff, R.B., Baretto, F., Pizzolato, Т.M., Sirtori, C. (2016). Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry. Talanta, 154:38-45.
Asakawa, D., Uemura, M., Sakiyama, T., Yamano, T. (2018). Sensitivity enhancement of aminoglycosides in hydrophilic interaction liquid chromatography with tandem mass spectrometry by post-column addition of trace sodium acetate in methanol. Food Additives and Contaminants: Part A, 35:1116-1126.
Bohm, D.A., Stachel, C.S., Gowik, P. (2013). Validation of a method for the determination of aminoglycosides in different matrices and species based on an in-house concept. Food Additives and Contaminants, 30:1037-1043.
Butler, M.M., Waidyarachchi, S.L., Connolly, K.L., Jerse, A.E., Chai, W., Lee, R.E., Kohlhoff, S.A., Shinabarger, D.L., Bowlin, T.L. (2018). Aminomethyl spectinomycins as therapeutics for drug-resistant gonorrhea and chlamydia coinfections. Antimicrob Agents Chemother, 62:e00325.
http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32010R0037
Council Directive 96/23/EC on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. (1996). Official Journal of the European Union, 125:10-32.
Council Regulation (EC) N° 2377/90 of 26 June 1990: laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. (1990) Official Journal of European Community, 224:14.
Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. (2002). Official Journal of the European Commission, 221:8-28
Guoa, Y., Xiec, X., Diaoa, Z., Wanga, Y., Wang, B., Xiea, K., Wanga, X., Zhanga, P. (2021). Detection and determination of spectinomycin and lincomycin in poultry muscles and pork by ASE-SPE-GC–MS/MS. Journal of Food Composition and Analysis, 101:103979.
European Medicines Agency (2019). Sales of veterinary antimicrobial agents in 31 European countries in 2017. Trends from 2010 to 2017 Ninth ESVAC report. European Medicines Agency, London.
Lisicyn, A.B. (2015). Myasnaya promyshlennost'. Enciklopedicheskij slovar'/A.B. Lisicyn [i dr.]. Moskva: VNIIMP, 256 s. (In Russian) Maron, D.F., Smith, T.J., Nachman, K.E. (2013). Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Global Health, 9:48.
Miahka K.S., Kostiuk M.V., Tkachuk S.A. (2018). Metodychni rekomendatsii z kilkisnoho vyznachennia spektinomitsynu v molotsi za dopomohoiu test-systemy dlia imunofermentnoho analizu Kwinbon Biotech Spectinomycin (Cat. No.: KA02702H). K., DNDILDVSE. (In Ukrainian).
Myagka, K.S., Tkachuk, S.A., Mezhenska, N.A. (2018). RidaScreen test system for Nitrofuran (AOZ) and Chloramphenicol screening in the honey. Ukrainian Journal of Ecology, 8:892-897.
Verrette, L, Fairbrother, J.M., Boulianne, M. (2019). Effect of Cessation of ceftiofur and substitution with lincomycin-spectinomycin on extended-spectrum-β-lactamase/ampc genes and multidrug resistance in escherichia coli from a canadian broiler production pyramid. Applied Environment Microbiology, 85:e00037-19.
Wang, B., Wang, Y., Xie, X., Diao, Z., Xie, K., Zhang, G., Zhang, T., Dai, G. (2020). Quantitative analysis of spectinomycin and lincomycin in poultry eggs by accelerated solvent extraction coupled with gas chromatography tandem mass spectrometry. Foods, 18:651.
Wang, X., Yang, S., Li, Y., Zhao, W., Zhang, Y., Huang, J., Wang, P., Wu, C., Zhou, J. (2018). Optimization and application of parallel solid-phase extraction coupled with ultra-high performance liquid chromatography-tandem mass spectrometry for the determination of 11 aminoglycoside residues in honey and royal jelly. Journal of Chromatography A, 1542:28-36.
Wua, Q., Gao, X., Abu Bak, M., Dapen, S., Yanfe, P., Dongmei, T., Haihong, C., Guyue, H., Zhenli, C., Zonghui, L., Wanga, Y.Y. (2019). Rapid multi-residue screening of antibiotics in muscle from different animal species by microbiological inhibition method. Microchemical Journal, 152:104417.
Holthoon van, F.L., Essers, M.L., Mulder, P.J., Stead, S.L. Caldow, M., Ashwin, H.M., Sharman, M.A. (2008). Generic method for the quantitative analysis of aminoglycosides (and spectinomycin) in animal tissue using methylated internal standards and liquid chromatography tandem mass spectrometry. Analytica Chimica Acta, 637:135-143.
Yanovych, D.V., Zasadna, Z.S., Kislova, S.M., Pazderska, O.M., Maiba, N.A. (2014). Zastosuvannia imunofermentnoho metodu dlia skryninhu zalyshkovykh kilkostei veterynarnykh preparativ ta kontaminantiv u produktakh tvarynnoho pokhodzhennia Naukovo-tekhnichnyi biuleten Instytutu biolohii tvaryn i Derzhavnoho naukovo-doslidnoho kontrolnoho instytutu vetpreparativ ta kormovykh dobavok, 15:249-255 (In Ukrainian).
Author Info
K.S. Myagka1, S.A. Tkachuk2, I.V. Yatsenko3, K.O. Rodionova4*, M.V. Kostiuk1, N.V. Liniychuk1, V.P. Lyasota5, V.M. Zhylina3, I.L. Tsivirko3 and L.B. Savchuk62Kharkiv State ZooVeterinary Academy, Kharkiv, Ukraine
3Kharkiv State ZooVeterinary Academy, Kharkiv, Ukraine
4Odessa State Agrarian University, 13, Panteleimonovskaya St, Odessa, 65012, Ukraine
5Hygiene of Livestock Products and Pathological Anatomy named after Y. S. Zahaievskii, Ukraine
6Podolsk State Agrarian Technical University, Ukraine
Citation: Myagka, K.S., Tkachuk, S.A., Yatsenko, I.V., Rodionova, K.O., Kostiuk, M.V., Liniychuk, N.V., Lyasota, V.P., Zhylina, V.M., Tsivirko, I.L., Savchuk, L.B. (2021). Validation of quantitative determination method of spectinomycin in muscle samples by the immunoenzyme analysis method. Ukrainian Journal of Ecology, 11 (7), 33-38.
Received: 28-Jul-2021 Accepted: 17-Sep-2021 Published: 27-Sep-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
