Research Article - (2023) Volume 13, Issue 1
Update on the distribution and the status the Asian tiger mosquito (Aedes albopictus) on the population of Culicidae in the North-east of Algeria
R. Amna1, A. Yasmine1, M. Brahim2*, B. Mounir1, K. Khamsa1, R. Abdelhakim3, R. Kamel3 and O.M. Laid3Abstract
Aedes albopictus was collected from some regions in Northeast Algeria to have an understanding of the distribution and the type of breeding site that the species prefers in the area, throughout the years (2017-2020). Then an ovitrap method was conducted to learn more about this invasive species' behavior compared with most abundant species (Culex pipiens and Culiseta longiareolata). After revealing the arrival of the Asian tiger mosquito to the region of Annaba in 2017 during mosquito inventory, we also declared it for the first time in both Bouchegouf (Guelma) and Besbes (El Tarf). We determined that this species prefers urban artificial breeding sites. In Besbes (El Tarf), the ovitraps revealed Ae. albopictus as the most abundant over species Cx. pipiens and Cs.longiareolata (82.09%) (F2.69=7.14; P=0.002). Thus, this invasive and adaptive species could coexist with local mosquito species, it can even displace them.
Keywords
Aedes albopictus, Inventory, Ovitrap, Abundance, Northeast Algeria.
Introduction
The spread of arbovirus in the world is increasing due to many factors: demographic movement, economic globalization (Wu et al., 2017). These factors have a great impact on the introduction of new diseases and their victors to other parts of the world, such as the Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) (Girard, et al., 2020)
Ae albopictus is considered one of the 100 most invasive species in the world, presenting a significant expansion in many parts of the globe (Giatropoulos, et al., 2013; Badieritakis, et al., 2018). This species is a vector for dengue, chikungunya, yellow fever, Japanese encephalitis, and Zika virus (Giatropoulos et al., 2012; Kampen et al., 2013), and zoonoses, such as dirofilarioses (Giangaspero, et al., 2013), presenting a notable threat to public health. Apart from being a vector of viruses, Ae. albopictus causes serious nuisance to people. This dramatic expansion is an actual public health crisis in the 21st century. Prevention and control of vector-borne diseases’ emergence and resurgence represent a critical challenge for the global health system (Collantes, et al., 2014).
Ae.albopictus worldwide spread started in the Pacific and Indian islands, and reached North America in 1985, then South American nations in subsequent years. Ae albopictus only reached Europe in 1979, arriving in Albania presumably as eggs or larvae in used water-filled tires (Adhami and Reiter, 1998). It has now expanded through the Peri-Mediterranean area (Kraemer, et al., 2015), east to Georgia (Kutateladze, et al., 2016), and west to the remaining parts of southern Europe (Marabuto and Rebelo, 2018).
The first recorded sighting of Ae. albopictus on the African continent was reported in 1989 in the Republic of South Africa (Cornel and Hunt, 1991). The following year, the distribution of the Asian Tiger mosquito extended to Nigeria, according to Savage et al., and thereafter it increased rapidly to the coastal regions of Western and Central Africa (Savage, et al., 1992), and confirmed by (Fontenille and Toto, 2001).
After the first appearance of the Asian tiger mosquito in Algeria in 2010 by (Izri, et al., 2011), the Pasteur Institute of the Ministry of Health has published an awareness report to people asking them to inform them about the areas where this insect was found and also explain to them how to deal with this very aggressive species towards humans to avoid its spread and thus its bites (Benallal, et al., 2019).
Ae. albopictus was first described by Skuse in 1894 from specimens collected in the city of Calcutta on the Indian subcontinent (Huang, 1968). This mosquito, native to the forests of Southeast Asia, has now achieved the Americas, Africa, the Middle East, and Europe, largely due to human activities (Gratz, 2004).
Numerous studies have been carried out on mosquitoes in the North-east of Algeria, but until 2017, there was no mention of Ae. albopictus in any of them. Therefore, this paper aims to understand the current status of this species in terms of behavior and reproductive biology as well as its ecological impact in northeast Algeria.
Materials and Methods
An entomological study targeting the population of Culicidae in the extreme Northeast of Algeria covered Annaba, El Tarf, and Guelma, where the observation started in 2017. Since this area is known for its diverse biotopes. Especially wetlands, they are considered suitable for mosquito breeding. The study area is located on the southern shore of the Mediterranean basin, in the northeast of Algeria. Annaba and El Tarf are two coastal plain regions that have industrial and agricultural activities with economic and fishing harbours, while Guelma is an inland mountain region with agricultural activities. They enjoy a Mediterranean climate where precipitation is abundant and can be torrential, and it’s generally hot, especially from June to August.
This study had two parts. The first was conducted from 2017 to 2020 (May to September) to randomly survey Ae. albopictus in both natural and artificial breeding habitats to obtain eggs, larvae, adults’ abundance, using the dipping method (Awono-Ambéné and Robert, 1999) and a strainer for the aquatic stages, and using a net and vials to collect adults, covering the study area in Annaba (El Bouni 1, El Bouni 2, Oued Forcha, El-rym, Oued Dhab, Bouhdid, L’Orangerie, Zaafrania, and El Hadjar have the numbers from 1 to 8 in that order) (Fig. 1).
Fig 1: Position of sites visited in Annaba.
In the year 2021, we initiated the second part, using a method that was developed through the results of the first part, which is called "ovitraps". The use of this method began in 1965. Oviposition traps for Aedes populations were shown to be effective for larval research and studies on vector frequency (Fay and Eliason, 1966; Regis, et al., 2008).
We've put the ovitraps in specific sites (the accessibility, dense vegetation, less exposure to wind, and protected), and visited every two weeks (a total of 24 samplings per year) to collect the larvae, aiming to provide an attractive spawning site. These traps permanently contained water. They were located in an environment that is itself attractive to mosquitoes, and we chose for it three-points, each in a community: El Bouni (36°51,010'N; 007°43.900'E) in the region of Annaba; Besbes (36°29.902'N; 007°43.060'E) in the region of El Tarf; and Bouchegouf (36°42.140'N; 007°50.681'E) in the region of Guelma appears in Fig. 2.
Fig 2: Sites of the puted ovitraps.
The trap consists of a black plastic bucket with a volume of 10 liters (Marabuto and Rebelo, 2018) (Fig. 3). These were placed in house gardens, because the preferential lodgings of Ae. albopictus are unexposed to the sun and the wind (Hawley, 1988). As well as following the population of Ae. albopictus, this method was a tool to detect other species of mosquitoes that are able to cohabit with it, and that led to a Comparative study.

Fig 3: Model of the nest trap used.
Statistical analysis
Several data were calculated, namely: the means, the standard deviation and the extremes for the various parameters. We used the kruskal-wallis test (One Way Anova) to compare between several means to determine if there are differences between sites (Bouchegouf, Besbes and El Bouni) and species (Ae. albopictus, Cx. pipiens, and Cs. longiareolata). For all these parameters, we used the STATISTIX software, version 8.
Results
Appearance and distribution of Ae. albopictus in Annaba
In the summer of 2017, during a mosquito inventory, we trapped an adult female of Ae. albopictus in the region of Annaba, which was taken to the laboratory to be identified using the software of the European identification key for mosquitoes (Schaffner, et al., 2001). This was followed by several complaints from people in the area about a series of mosquito bites causing infections, assuming that it was the Asian tiger mosquito (Fig. 4).
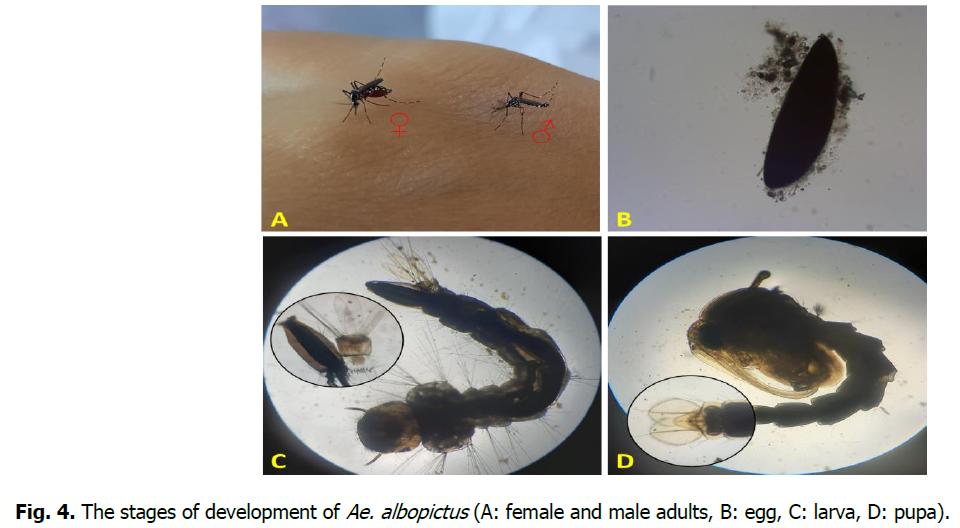
Fig 4: The stages of development of Ae. albopictus (A: female and male adults, B: egg, C: larva, D: pupa).
After the initial sighting of Ae. albopictus that occurred in 2017 (only one adult female), the research team decided to focus its work on this new species of mosquito that appeared in the region of Annaba. The obtained results show the population development throughout the past four years in the area (in 2017 we found only 1 specimen but we collected 608 specimens in 2020) (Table 1).
| Sites | GPS coordinats | 2017 | 2018 | 2019 | 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | L | A | E | L | A | E | L | A | E | L | A | ||
| El Bouni 1 | 36°50.977'N 007°44.687'E | 1 | 241 | 211 | 8 | 17 | 34 | 132 | 61 | 2 | |||
| El Bouni 2 | 36°50.872'N 007°44.550'E | 96 | 79 | 322 | 194 | 5 | 279 | 87 | 1 | ||||
| Oued forcha | 36°54.548'N 007°44.096'E | 520 | 246 | ||||||||||
| El-rym | 36°52.837'N 007°43.659'E | 1 | |||||||||||
| Oued dhab | 36°53.522'N 007°44.516'E | 40 | |||||||||||
| Bouhdid | 36°53.299'N 007°42.682'E | 6 | |||||||||||
| L’orangerie | 36°54.127'N 007°44.558'E | 26 | |||||||||||
| Zaafrania | 36°45.649'N 007°45.189'E | 712 | 528 | 12 | |||||||||
| Elhadjar | 36°46.805'N 007°43.694'E | 91 | 17 | 2 | |||||||||
| Total | 1 | 337 | 290 | 9 | 859 | 474 | 5 | 1442 | 917 | 18 | |||
Table 1. Ae. albopictus specimen collected from 2017 to 2020 in the region of Annaba. (E: Egg; L: larva; A: Adult).
This last show the total specimens collected from surveyed sites. We recorded the highest specimens in Zaafrania (712 eggs, 528 larvae, and 12 adults) in the year 2020. Also, the number of sites infested with Ae. albopictus had increased to nine sites in the region of Annaba. By the end of 2020, we sampled a total of 1442 eggs, 917 larvae, and 18 adults from 7 infested sites. Also, both El Bouni 1 and El Bouni 2 were constant sites that had been positive with Ae. albopictus throughout the years.
Description of the breeding sites
Of all the sites visited in this inventory Ae.albopictus was only found in artificial breeding habitats made of plastic, metal, and clay that come in many forms, such as buckets, pots, tanks, and discarded tires. These breeding sites are also of different sizes; 5, 10, 20, and 200 liters. We have noticed that breeding sites of Ae. albopictus are in gardens with a high density of vegetation, specifically under trees and in less windy areas (Fig. 5).

Fig 5: The different type of artificial breeding sites of Ae. albopictus.
Culicidae populations structure collected in all regions of the Northeast of Algeria
In the years 2019 and 2020, we noticed an increase in Ae. albopictus populations in the area where they started to cohabit sites with Cx. pipiens and Cs. longiareolata. Therefore, we wanted to know the status of these species by following the ovitraps method and also to know if Ae. albopictus had reached the surrounding areas of Annaba (Guelma and El Tarf). So, we chose three points for the ovitraps (El Bouni-Annaba; Besbes-El Tarf; and Bouchegouf-Guelma).
The results of the total individuals collected during the whole study period of each species (Cx. pipiens, Cs. longiareolata, and Ae. albopictus) of the ovitraps represented in Fig. 6. We sampled 6798 specimens. Among all collections, 1109 (16.31%) specimens were of Cx. pipiens; 2069 (30.44%) belonged to Cs. longiareolata; and 3620 (53.25%) were of Ae. albopictus.
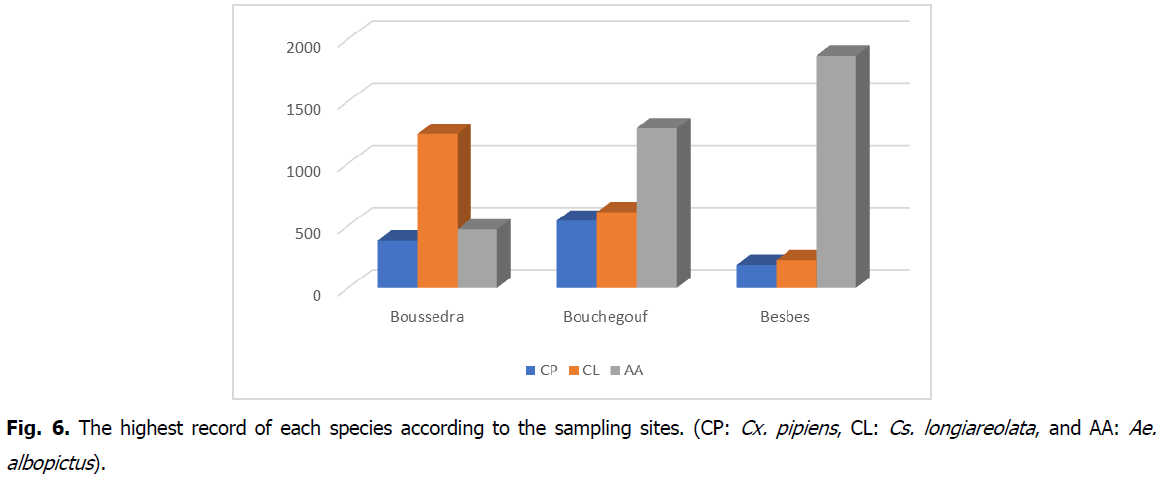
Fig 6: The highest record of each species according to the sampling sites. (CP: Cx. pipiens, CL: Cs. longiareolata, and AA: Ae. albopictus).
Table 2 represents the total samples of each species from different sites. Roughly over half of the samples collected from El Bouni belong to Cs.longiareolata (59.18%), whereas 22.51% were Ae albopictus, and 18.31% belong to Cx. pipiens. In Bouchegouf, we obtained 52.73% of Ae. albopictus, with 25.03% of Cs.longiareolata and 22.24% of Cx;pipiens. While Besbes' simples revealed 82.09% Ae. albopictus and only 9.77% and 8.14% Cs.longiareolata and Cx.pipiens, respectively, These results indicated that Besbes didn’t only have the highest record of Ae. albopictus simples but also the highest record of all three species.
| CP | CL | AA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Specimens |
% per site | Max Density | Number of Speimens | % per Site | Max Density | Number of Specimen | % per Site | Max Density | |
| El Bouni | 383 | 18.31 | 21.8 | 1238 | 59.18 | 41.5 | 471 | 22.51 | 14.1 |
| Bouchegouf | 541 | 22.24 | 18.5 | 609 | 25.03 | 15.9 | 1283 | 52.73 | 41.5 |
| Besbes | 185 | 8.14 | 7.3 | 222 | 9.77 | 12.4 | 1866 | 82.09 | 45.9 |
| Total | 1109 | 2069 | 3620 | ||||||
| Mean | 15.87 | 23.27 | 33.83 | ||||||
| % per species | 16.31 | 30.44 | 53.25 | ||||||
Table 2. Number, percentages and maximum density of species collected in the three sites. (CP: Cx. pipiens, CL: Cs. longiareolata, and AA: Ae. albopictus).
The average number of larvae of Cx. Pipiens was the highest in Bouchegouf (22.542 ± 48.992) as well as the average of Cs. longeariolata in El Bouni (51.583 ± 104.678) while the average of Ae. Albopictus was the highest in Besbes (77.75 ± 127.108) (Table 3).
| n:mean ± Standar error (Min-Max) | Kuskal-Wallis | |||
|---|---|---|---|---|
| B | F | S | ||
| CP | 15.95 ± 49.735 (0-218) |
22.542 ± 48.992 (0-185) |
7.708 ± 21.282 (0-73) |
F2.69=0.74; P=0.481 |
| CL | 51.583±104.678 (0-415) |
25.375±45.282 (0-159) |
9.25±27.173 (0-124) |
F2.69= 0.59; P= 0.556 |
| AA | 19.625±41.114 (0-146) |
53.458 ±111.839 (0-415) |
77.75 ±127.108 (0-459) |
F2.69= 1.24; P= 0.296 |
| Kuskal- Wallis |
F2.69= 0.41; P= 0.665 | F2.69= 0.71; P= 0.495 | F2.69= 7.14; P= 0.002 | - |
Table 3. Number of specimen of each species (CP, CL, and AA) in each site (B, F, and S). Species: (CP: Cx. pipiens, CL: Cs. longeariolata, AA: Ae. albopictus) Sites: (B: Boussedra, F: Bouchegouf S: Besbes).
The data analysis of species in all sites using Kruskal-Wallis test, in which Cx. pipiens (F2.69=0.74; P=0.481), Cs.longeariolata (F2.69=0.59; P=0.556), and Ae. albopictus (F2.69=1.24; P=0.296) showed no significant difference between the average number of larvae of each species in the three sites.
On the other hand, the average number of larvae in each site where we have El Bouni and Bouchegouf (F2.69=0.41; P=0.665), (F2.69=0.71; P=0.495) successfully with no significant difference between the species in both sites, but in Besbes (F2.69=7.14; P=0.002) the average difference between the species was highly significant (Table 3).
In all three surveys, the larvae’s presence was from April to October, and then it almost completely disappeared from November to March. Fig. 7 shows the increase of the population of the three species throughout the time in each site (A, B, and C) and also the total of all sites (D).
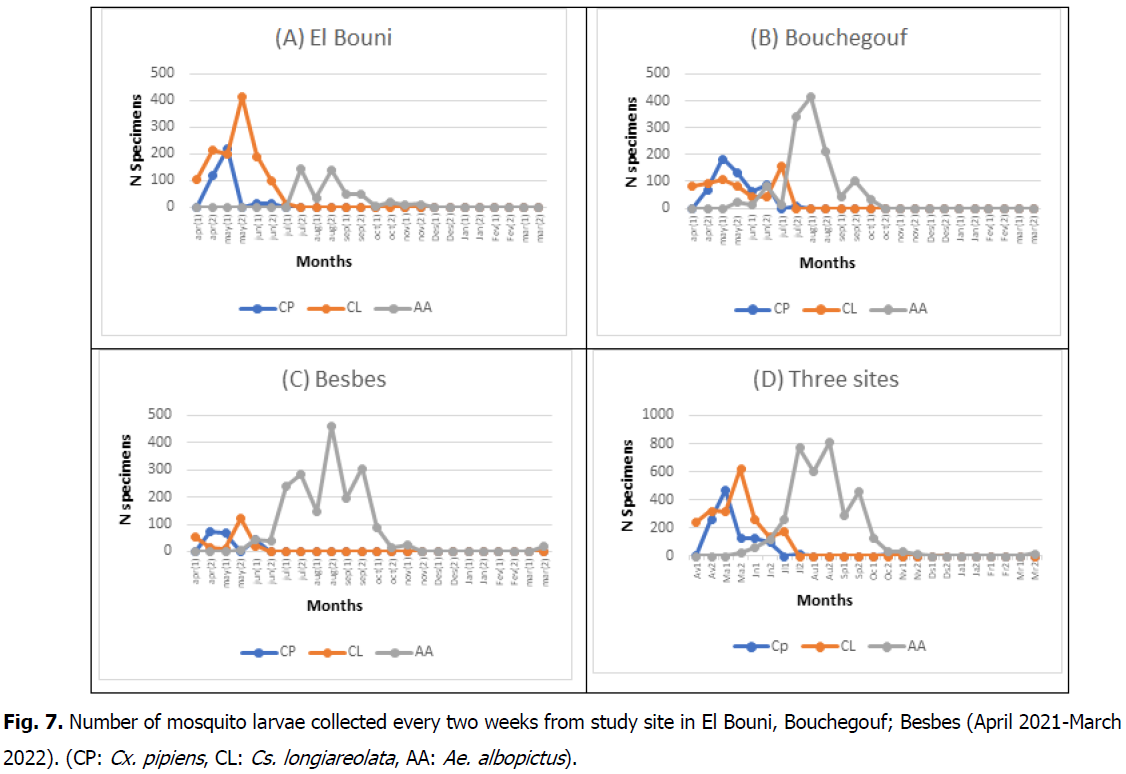
Fig 7: Number of mosquito larvae collected every two weeks from study site in El Bouni, Bouchegouf; Besbes (April 2021-March 2022). (CP: Cx. pipiens, CL: Cs. longiareolata, AA: Ae. albopictus).
In El Bouni, Cx.pipiens (218 specimens in early May) and Cs. Logiareolata (415 larvae in late May) installed in the site from early April to July. Moreover, Ae. albopictus appears at the site later (late July), after almost both Cx. pipiens and Cs. longiareolata completely disappear, having the site for itself till late November (459 larvae in late August). In Bouchegouf also, Cx. pipiens (185 specimens in early May) and Cs. longiareolata (159 specimens early July) shared the site from April to July, but only late June Ae. albopictus came along till late July to have its peak later (415 early August) and disappear in November. In Besbes, both Cx. pipiens and Cs. longiareolata had an important ecological valance on the site (from April to early June) and once Ae. albopictus was found, it had the site till early November (459 specimens late August).
The D graph of the Fig. 7. demonstrates the number of mosquito larvae of all species at the three sites combined, where we can see clearly that the presence in large numbers was for Cx. pipiens in the early season, for Cs longiareolata at mid-season and by the end of the season it was only Ae. albopictus, even though they all shared what we call the transition period.
The specimens collected showed that Ae. albopictus is capable to cohabit breeding sites with several species (especially from June to July), even at times when we found all three of them together. Then it’s the most abundant in the sites for months (July to September), more specifically during summer (the dry and hot period).
Discussion and Conclusion
Ae. albopictus is a recent invasive species in the Mediterranean region (Adhami and Reiter, 1998). Several factors contributed to the rapid spread of this mosquito species. Among the most important are the resistance of its desiccated, dormant eggs and their efficient passive transport around the world, often in used tires (Tatem, et al., 2006) or in imported plants (Benallal, et al., 2019).
In Algeria, the first observation of this species was in 2010 in the region of Tizi-Ouzou by Izri et al., to be the first report for the species in the country (Izri, et al., 2011). Then it was followed by other observations in other regions of the country, which were the region of Oran in 2015 by (Benallal, et al., 2019); Algiers in 2016 by (Benallal, et al., 2019; Merabti, et al., 2021) the region of Annaba in 2018 by (Arroussi, et al., 2021), and last in 2020 in the region of Souk Ahras by (K. Hamaidia and Soltani, 2021). Our research team notes the first observation of this species in the Annaba region in 2017 and in the regions of El Tarf and Guelma in 2021, as recorded in our current work, which confirms that it’s now almost spread all over Northeast Algeria.
This mosquito used to breed in natural habitats, especially in tree holes, leaf axils, rock pools, and similar sites (Hawley, 1988). Ae. albopictus is omnipresent and readily adapts to diverse environmental conditions in tropical and temperate regions (Rai, 1991). Our inventory (2017-2020) results conclude that Ae. albopictus is found only in the urban environment. We never come across this species in natural sites, only in artificial ones (buckets, barrels, tanks, cups, tires...). It is confirmed by (Swaddiwudhipong, et al., 1992; Mellander, et al., 2015) that the shortage of water supply during the dry season can increase the number of storage containers in the community, plus the discarded containers and solid wastes filled with rainwater can create more breeding habitats because Ae. albopictus prefers artificial sites in urban areas. Gubler et al., and Delatte et al have interpreted this phenomenon as widespread deforestation, climate change, and an increase in global trade have forced this mosquito worldwide to adapt to breeding in artificial container habitats (Gubler, et al., 2001; Delatte, et al., 2008).
Ovitraps provide useful data on the spatio-temporal distribution of mosquitoes because this monitoring allows one to check the presence and density of the vector at a local scale (dos Santos Nunes, et al., 2011; Fischer, et al., 2017). Although previous inventories of mosquitoes in the area confirm the presence of 18 species of mosquitoes (Houmani et al., 2017; Benmalek et al., 2018; Arroussi et al., 2021) in the region of Annaba. Out of fifty-three species prospered in Algeria from 1903 to 2021 according to (Merabti et al., 2021), our ovitraps attract only three species (Cx.pipiens, Cs. longiareolata, and Ae. albopictus).
Our results indicate that the resident species of Ae. albopictus are Cx. pipiens and Cs. longiareolata, whereas several works talk about Ae. aegypti as a resident species, like those of (Simard, et al., 2005; Kamgang, et al., 2010). This is the first study that refers to the coexistence of Ae. albopictus with Cx. pipiens and Cs. longiareolata.
Cx. pipiens was the most abundant species based on most survey studies in Algeria, like (Lafri, et al., 2014; H. Hamaidia and Berchi, 2018; Arroussi, et al., 2021; K. Hamaidia and Soltani, 2021). Contrary to what we found in our ovitraps, after the arrival of Ae. albopictus in the area, we can see that this last is the most abandoned. Moreover, Cx. pipiens and Cs. longiareolata showed, in previous studies by (Benhissen, et al., 2017; Asloum, et al., 2021) high ecological plasticity and habitat coexistence. Now that behavior is being affected by the appearance of Ae. albopictus in the region when it comes to the artificial sites but this remark can’t be accrued when we talk about natural sites. A study in the Central African Republic showed that the introduced species of Ae. albopictus predominated over Ae. aegypti, according to (Kamgang, et al., 2010). The coincident decline in several Cx. pipiens and Cs. longeariolata populations that occurred after Ae. albopictus invasions may have resulted from competitive displacement, which gave it an advantage over local species, as mentioned by (Livdahl, 1993; Braks, et al., 2004) in the competition between Ae. aegypti and Ae. albopictus, where this last invaded Rio de Janeiro (Brazil). Where competition is most intense, Ae. aegypti is eliminated (Juliano, 1998); this is what we see in Besbes (El Tarf), where Ae. albopictus is dominant on indigenous species Cx. pipiens and Cs. Longiareolata (we assume because it is in a region with very high agglomeration and placed is a garden with high diversity of vegetation). Therefore, it is an invasive and adaptive species, coexisting with local mosquito species that could even displace them.
According to the results obtained, Ae. albopictus will likely continue to spread to the rest of the country. (Vidal et al., 2012) considered its control difficult due to its rapid reproduction and ability to adapt to different environments, also for conditioning more breeding habitats for their victors as a result of anthropogenic activities.
Acknowledgements
We would like to express our sincere appreciation to all the people who have contributed directly or indirectly to this study. Many thanks are due to the laboratory staff of the Ecology laboratory of marine and Coastal environments (EMMAL) at Badji Mokhtar University, The Laboratory of Genetic, Biotechnology and Valorization of Bioresources (LGBVB), University of Biskra, for their helps.
References
Adhami, J., Reiter, P. (1998). Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. Journal of the American Mosquito Control Association, 14:340-343.
Arroussi, D.E.R., Bouaziz, A., Boudjelida, H. (2021). Mosquito survey reveals the first record of Aedes (Diptera: Culicidae) species in urban area, Annaba district, Northeastern Algeria. Poland Journal of Èntomology, 90:14-26.
Google Scholar, Crossref, Indexed at
Asloum, A.Y., Benhissen, S., Habbachi, W., Habbachi, S., Hedjouli, Z., Bouselama, Z., Tahraoui, A. (2021). Preliminary inventory and general aspect of the distribution of Culicidae species in the Steppe region (M'sila, Algeria). Journal of Bioresource Management, 8:8.
Awono-Ambéné, H.P., Robert, V. (1999). Survival and emergence of immature Anopheles arabiensis mosquitoes in market-gardener wells in Dakar, Senegal. Parasite, 6:179-184.
Badieritakis, Ε., Papachristos, D., Latinopoulos, D., Stefopoulou, Α., Kolimenakis, Α., Bithas, K., Michaelakis, Α. (2018). Aedes albopictus (Skuse, 1895) (Diptera: Culicidae) in Greece: 13 years of living with the Asian tiger mosquito. Parasitology Research, 117:453-460.
Benallal, K.E., Garni, R., Bouiba, L., Harrat, Z. (2019). First detection of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Algiers, the capital city of Algeria. Journal of Arthropod-Borne Diseases, 13:420.
Benhissen, S., Habbachi, W., Ouakid, M.L. (2017). Biodiversity and distribution of mosquitoes (Diptera: Culicidae) in the oases of the Biskra region (south-eastern Algeria). Algerian Journal of Arid Environment, 7:96-101.
Benmalek, L., Bendali-Saoudi, F., Soltani, N. (2018). Inventory and distribution of mosquitoes (Diptera; Culicidae) of the Burgas lakes (Northeast Algeria). Journal of Entomology and Zoology Studies, 6:838-843.
Braks, M.A.H., Honório, N.A., Lounibos, L.P., Lourenço-de-Oliveira, R., Juliano, S.A. (2004). Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Annals of the Entomological Society of America, 97:130-139.
Collantes, F., Delgado, J.A., Alarcón-Elbal, P.M., Delacour, S., Lucientes, J. (2014). First confirmed outdoor winter reproductive activity of Asian tiger mosquito (Aedes albopictus) in Europe. In Anales de Biología, Servicio de Publicaciones de la Universidad de Murcia, 36:71-76.
Cornel, A.J., Hunt, R.H. (1991). Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. Journal of the American Mosquito Control Association, 7:107-108.
Delatte, H., Paupy, C., Dehecq, J.S., Thiria, J., Failloux, A.B., Fontenille, D. (2008). Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite (Paris, France), 15:3-13.
Google Scholar, Crossref, Indexed at
dos Santos Nunes, L., Trindade, R.R., Souto, R.N. (2011). Avaliação da atratividade de ovitrampas a Aedes (Stegomyia) aegypti Linneus (Diptera: Culicidae) no bairro Hospitalidade, Santana, Amapá. Biota Amazônia, 1:26-31.
Fay, R.W., Eliason, D.A. (1966). A preferred oviposition site as a surveillance method for Aedes aegypti. Mosquito News.
Fischer, S., De Majo, M.S., Quiroga, L., Paez, M., Schweigmann, N. (2017). Long-term spatio-temporal dynamics of the mosquito Aedes aegypti in temperate Argentina. Bulletin of Entomological Research, 107:225-233.
Fontenille, D., Toto, J.C. (2001). Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerging Infectious Diseases, 7:1066.
Google Scholar, Crossref, Indexed at
Giangaspero, A., Marangi, M., Latrofa, M.S., Martinelli, D., Traversa, D., Otranto, D., Genchi, C. (2013). Evidences of increasing risk of dirofilarioses in southern Italy. Parasitology Research, 112:1357-1361.
Giatropoulos, A., Papachristos, D.P., Kimbaris, A., Koliopoulos, G., Polissiou, M.G., Emmanouel, N., Michaelakis, A. (2012). Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitology Research, 111:2253-2263.
Giatropoulos, A., Pitarokili, D., Papaioannou, F., Papachristos, D.P., Koliopoulos, G., Emmanouel, N., Michaelakis, A. (2013). Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae). Parasitology Research, 112:1113-1123.
Girard, M., Nelson, C.B., Picot, V., Gubler, D.J. (2020). Arboviruses: A global public health threat. Vaccine, 38:3989-3994.
Google Scholar, Crossref, Indexed at
Gratz, N.G. (2004). Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology, 18:215-227.
Gubler, D.J., Reiter, P., Ebi, K.L., Yap, W., Nasci, R., Patz, J.A. (2001). Climate variability and change in the United States: potential impacts on vector-and rodent-borne diseases. Environmental Health Perspectives, 109:223-233.
Hamaidia, H., Berchi, S. (2018). Systematic and Biotypical study of the family Culicidae (Diptera-Nematocera) in the region of Tebessa (Algeria). International Journal of Mosquito Research, 5:39-46.
Hamaidia, K., Soltani, N. (2021). New report of Aedes albopictus in Souk Ahras, Northeast Algeria. Biodiversitas Journal of Biological Diversity.
Hawley, W.A. (1988). The biology of Aedes albopictus. Journal of the American Mosquito Control Association. Supplement, 1:1-39.
Houmani, M., Bendali-Saoudi, F., Soltani, N. (2017). Inventory of Culicidae in the region of El Taref (North-east Algeria). Journal of Entomology and Zoology Studies, 5:263-267.
Huang, Y.M. (1968). Neotype designation for Aedes (Stegomyia) albopictus (Skuse)(Diptera: Culicidae). Smithsonian Institution Washington DC.
Izri, A., Bitam, I., Charrel, R.N. (2011). First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clinical Microbiology and Infection, 17:1116-1118.
Google Scholar, Crossref, Indexed at
Juliano, S.A. (1998). Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition?. Ecology, 79:255-268.
Kamgang, B., Happi, J.Y., Boisier, P., Njiokou, F., Hervé, J.P., Simard, F., Paupy, C. (2010). Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Medical and Veterinary Entomology, 24:132-141.
Kampen, H., Kronefeld, M., Zielke, D., Werner, D. (2013). Further specimens of the Asian tiger mosquito Aedes albopictus (Diptera, Culicidae) trapped in southwest Germany. Parasitology Research, 112:905-907.
Kraemer, M.U., Sinka, M.E., Duda, K.A., Mylne, A.Q., Shearer, F.M., Barker, C.M., Hay, S.I. (2015). The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife, 4:e08347.
Kutateladze, T., Zangaladze, E., Dolidze, N., Mamatsashvili, T., Tskhvaradze, L., Andrews, E.S., Haddow, A.D. (2016). First record of Aedes albopictus in Georgia and updated checklist of reported species. Journal of the American Mosquito Control Association, 32:230-233.
Lafri, I., Bitam, I., Beneldjouzi, A., Ben Mahdi, M.H. (2014). An inventory of mosquitoes (Diptera: Culicidae) in Algeria. Bulletin Society of Zoology France, 139:255-261.
Livdahl, T. (1993). Competition and egg-hatch inhibition among tire-breeding mosquitoes. Technical Report. Florida Department of Agriculture and Consumer Services, Bureau of Entomology and Pest Control, Jacksonville FL.
Marabuto, E., Rebelo, M.T. (2018). The Asian tiger mosquito, Aedes (Stegomyia) albopictus (Skuse), a vector of dengue, chikungunya and zika viruses, reaches Portugal (Diptera: Culicidae). Zootaxa, 4413:197-200.
Google Scholar, Crossref, Indexed at
Mellander, C., Lobo, J., Stolarick, K., Matheson, Z. (2015). Night-time light data: A good proxy measure for economic activity?. PloS One, 10:e0139779.
Merabti, B., Boumaza, M., Ouakid, M., Carvajal, T.M., Harbach, R.E. (2021). An updated checklist of the mosquitoes (Diptera: Culicidae) present in Algeria, with assessments of doubtful records and problematic species. Zootaxa, 5027:515-545.
Rai, K.S. (1991). Aedes albopictus in the Americas. Annual Review of Entomology, 36:459-484.
Regis, L., Monteiro, A.M., Melo-Santos, M.A.V.d., Silveira Jr, J.C., Furtado, A.F., Acioli, R.V., Ribeiro Jr, P.J. (2008). Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: basis for surveillance, alert and control system. Memórias do Instituto Oswaldo Cruz, 103:50-59.
Savage, H.M., Ezike, V.I., Nwankwo, A.C., Spiegel, R., Miller, B.R. (1992). First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. Medicine Hygine, 25:318-325.
Schaffner, F., Angel, G., Geoffroy, B., Hervy, J.P., Rhaiem, A., Brunhes, J. (2001). Les moustiques d’Europe: logiciel d’identification et d’enseignement= The mosquitoes of Europe: an identification and training programme. IRD Editions and EID Méditerranée: Montpellier, France.
Simard, F., Nchoutpouen, E., Toto, J.C., Fontenille, D. (2005). Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. Journal of Medical Entomology, 42:726-731.
Swaddiwudhipong, W., Chaovakiratipong, C., Nguntra, P., Koonchote, S., Khumklam, P., Lerdlukanavonge, P. (1992). Effect of health education on community participation in control of dengue hemorrhagic fever in an urban area of Thailand. Southeast Asian Journal of Tropical and Medical Public Health.
Tatem, A.J., Hay, S.I., Rogers, D.J. (2006). Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences, 103:6242-6247.
Vidal, P.O., Carvalho, E., Suesdek, L. (2012). Temporal variation of wing geometry in Aedes albopictus. Memórias do Instituto Oswaldo Cruz, 107:1030-1034.
Wu, T., Perrings, C., Kinzig, A., Collins, J.P., Minteer, B.A., Daszak, P. (2017). Economic growth, urbanization, globalization, and the risks of emerging infectious diseases in China: A review. Ambio, 46:18-29.
Author Info
R. Amna1, A. Yasmine1, M. Brahim2*, B. Mounir1, K. Khamsa1, R. Abdelhakim3, R. Kamel3 and O.M. Laid32Laboratory of Genetic, Biotechnology and Valorization of Bioresources (LGBVB), University of Biskra, Algeria
3Laboratory of Biology, Water and Environment (LBEE), the 8 Mai 1945 University, BP. 401, 24000, Guelma, Algeria
Citation: Amna, R., Yasmine, A., Brahim, M., Mounir, B., Khamsa, K., Abdelhakim, R., Kamel, R., Laid, O.M. (2023). Update on the distribution and the status the Asian tiger mosquito (Aedes albopictus) on the population of Culicidae in the North-east of Algeria. Ukrainian Journal of Ecology. 13:17-27.
Received: 21-Dec-2022, Manuscript No. UJE-22-84349; , Pre QC No. P-84349; Editor assigned: 23-Dec-2022, Pre QC No. P-84349; Reviewed: 05-Jan-2023, QC No. Q-84349; Revised: 11-Jan-2023, Manuscript No. R-84349; Published: 18-Jan-2023, DOI: 10.15421/2023_420
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
