Research Article - (2021) Volume 11, Issue 2
The role of macrophytes in waterfowl reproduction
N. Hrynevych1, M. Prychepa2*, Yu. Kovalenko2, O. Vodianitskyi2, M. Svitelskyi3, O. Fotin4, L. Zahorui1, V. Zharchynska1, B. Gutyj5*, S. Kulish6, V. Honcharenko3, T. Velesyk7, R. Sachuk7, Ya. Stravsky8 and N. Boltyk9Abstract
The formation of waterfowl communities was considered regards the overgrowth area in the lakes with higher aquatic plants. We found that water bodies with overgrowth of macrophytes by 33 and 50% had high biodiversity. When water bodies are overgrown by 23%, the highest number of nesting birds was observed, whereas when overgrown is 5%, the number of birds is much less. The same happens when water bodies are overgrown up to 90%. We revealed that the optimal lake overgrowth for the existence and reproduction of waterfowl and waterbirds was 33% and 50%, confirmed by the Shannon diversity indices (2.4 and 2.07, respectively). The relationship between the percentage of overgrowing and nesting birds number was also confirmed. We identified four bird species that breed successfully regardless of reservoir overgrowth, while nine bird species need special conditions. Our results can be implemented in the engineering, reclamation, and recreational activities in the municipal water bodies. Also, our data can be used to increase the biological diversity and productivity of water bodies.
Keywords
waterbirds, macrophytes, overgrowth percentage, overgrowth of water bodies, biodiversity.
Introduction
Most species are adapted to live in freshwater ecosystems, but as a result of anthropogenic activities, natural relationships often break down (Kofonov et al., 2020; Honcharova et al., 2021). This leads to an imbalance in ecosystems (Bashchenko et al., 2020; Borshch et al., 2020; 2021; Kalaitan et al., 2021; Butsiak et al., 2021; Saranchuk et al., 2021) and loss of habitat, which are necessary for the existence and reproduction of aquatic and semi-aquatic animals (Prysiazhniuk et al., 2019; Rudenko et al., 2019; Kalyn et al., 2020).
These processes are often accompanied by disruption or even destruction of trophic links, which can contribute to the spread of certain species - potential distributors of diseases and intermediate hosts of some parasites (Thomaz & da Cunha, 2020). The maintenance of the normal and stable functioning of freshwater systems is going on the groupings of macrophytes (Kawatsu et al., 2015). Which provide feeding and spawning ground for fish and invertebrates. Together they constitute the biological diversity of water bodies. The importance of macrophytes also lies in the proper maintenance of the sanitary and hydrochemical state of water bodies. The intensification of eutrophication, water pollution, and toxic compounds of higher aquatic plants is essential for water purification. Simultaneously, urban water bodies often undergo coastline transformations, particularly during the creation of recreational zones, accompanied by the destruction of reed groups that leads to an imbalance in ecosystems (Stefanidis et al., 2019). This problem is widespread in the cities of Ukraine; a similar situation can be traced in Greece and many other European countries (Svitok et al., 2016; Zhang et al., 2017). Thus, the arrangement of urban infrastructure requires accounting and knowledge about the minimum permissible percentage of natural resources, which can provide natural processes in the reservoir. This percentage can be determined by the presence or absence of breeding pairs of birds. Birds have a high species diversity, and in water bodies often occupy the top of the food chain; they quickly respond to changes in the environment. That is why they are used as reliable markers of the general state of the ecosystem under anthropogenic pressure (Klaassen & Nolet, 2007).
The work aimed to analyze the nesting groups of waterfowl in reservoirs with different percentages of overgrowth with higher aquatic plants.
Materials and Methods
The research was carried out during 2020 on the reservoirs of separate fishery ponds in Kyiv and Bila Tserkva. The choice is due to its location near the city and natural connections between macrophytes and birds. The percentage of overgrowth in water bodies was assessed by mapping the study area with a quadcopter. The counts of waterfowl and waterbirds were carried out by mapping with the subsequent categorization of the encounters, taking into account the breeding pairs with the spatial reference of all registrations on the precinct (Inventory methods .., 1999). The survey of habitats suitable for nesting was carried out on foot (Bibbi et al., 2000). On separate intervals, the data on the spring abundance of ducks, grebes, coots, and moorhen correlated with counting the results (late June and early July). Broods were counted in the morning and/or evening, when the birds are most active (Banik & Dzkamirzoyev, 2004).
To determine the composition and abundance of avifauna, dual control was used (two accounting during the season, particularly the 2nd half of May and the 1st half of June). The abundance was estimated based on the count of adult birds using individual approaches and taking into account the peculiarities of the nesting behavior of specific species. Non-nesting species were not taken into account. The presence and number of nests were checked periodically during the reproductive period. The reliability of nesting was determined following the criteria recommended by the Committee of the European Ornithological Atlas - EOAC (Breeding Bird Atlas of Europe, 1992). To take into account the Little Bittern (Ixobrychus minutus), shepherds, and warblers, we used mapping of nesting sites (based on the registration of votes) (Borowiec et al., 1981; Dombrowski, 1987). together with the study of suitable biotopes. Binoculars 12x and 10x and telescopes 20 * 40 were used for observations. The birds were identified according to “Birds of the fauna of Ukraine” (Fesenko & Bokotey, 2002). Calculate biodiversity indices according to (Aleksanov, 2017); statistical calculations were performed by methods (Svalov, 1977) using the program Statistica 12.
Characteristics of the study areas:
Belotserkovsky ponds and Borovetski ponds are not full-system fish farms located within the forest-steppe and filled with water from the river Protoka (Fig. 1 and 2), where Cyprinidae species grow to marketable sizes (carp Cyprinus carpio (L), grass carp Ctenopharyngodon idella (Valenciennes), silver carp hybrids Hypophthalmichthys hybr. The average pond area is 7.6 hectares.
Fig 1: Map of experimental ponds of Bila Tserkva fish farm
Fig 2: Map of experimental ponds Nyvky
Experimental farm “Nyvka” of the Institute of Fisheries of the National Academy of Agrarian Sciences of Ukraine located in Kyiv, within the Right Bank Polesie and Dnieper ecological corridor (Kazannyk et al., 2014). On the ponds, carp, Chinese crucian carp Carassius auratus (L), silver carp Hypophthalmichthys idella (Valenciennes), silver carp hybrids, pike Esox lucius (L), and perch Perca fluviatilis (L) are grown and multiplied. For the study, three ponds were selected, the average area of which was 9.7 hectares. We determined that the reservoirs were differed by plant composition and overgrowth degree.
Results and Discussion
We found that the basis of higher aquatic plants in the ponds of the Belotserkovsky fish farm was made up of broadleaf cattail Typha latifolia (L), common reed Phragmites australis (Cav.) Trin. ex Steud.) and sedge Carex sp. prevailed among underwater and floating plants Potamogeton sp. and Persicaria amphibia (L.).
Twenty-one nesting species of waterfowl belonging to the six rows were identified (Falconiformes, Charadriiformes, Anseriformes, Podicipediformes, Ciconiformes, Gruiformes).
On a reservoir with a minimum overgrown area of 5%, six breeding species were identified, in particular: 2 pairs of Common tern Sterna hirundo (L), eight pairs of Whiskered Tern Chlidonias hybrida (L), 17-25 pairs of Black-headed Gull Larus ridibundus (L) and four pairs of Coots Fulica atra (L) (Table 1). The presence of bird nests was associated with a small number of reed islands used by these birds as breeding habitats.
| No | Species name | % overgrowth of the reservoir | ||||
|---|---|---|---|---|---|---|
| 5% n=3 |
23% n=4 |
33% n=3 |
50% n=2 |
90% n=3 |
||
| 1 | Podiceps cristatus | 12 | 6 | 4 | ||
| 2 | Podiceps ruficollis | 2 | 1 | 1 | ||
| 3 | Podiceps nigricollis | 10 | 1 | 5 | ||
| 4 | Ixobrychus minutus | 1 | 5 | 6 | 6 | 2 |
| 5 | Botaurus stellaris | 1 | 4 | 1 | ||
| 6 | Ardea cinerea | 2 | ||||
| 7 | Egretta alba | 2 | ||||
| 8 | Ardea purpurea | 1 | ||||
| 9 | Cygnus olor | 1 | 1 | 1 | ||
| 10 | Anas platyrhynchos | 3 | 10 | 12 | 10 | 2 |
| 11 | Anas querquedula | 1 | ||||
| 12 | Aythya ferina | 1 | 7 | 2 | ||
| 13 | Gallinula chloropus | 1 | 6 | 9 | 6 | 2 |
| 14 | Porzana parva | 2 | 1 | 2 | ||
| 15 | Fulica atra | 4 | 23 | 25 | 20 | 2 |
| 16 | Rallus aquaticus | 2 | 2 | 3 | 1 | |
| 17 | Chlidonias niger | 6 | ||||
| 18 | Sterna hirundo | 2 | 2 | |||
| 19 | Chlidonias hybrida | 8 | 42 | |||
| 20 | Larus ridibundus | 17 | 30 | 65 | 42 | |
| 21 | Larus cachinnans | 2 | ||||
| Total: | 37 | 110 | 147 | 156 | 10 | |
Table 1. The number of nesting waterfowl and wetland birds depending on reservoir overgrowth (in pairs)
The pond with 23% overgrowth was characterized by the dominance of the common reed group. Under these conditions, 30 breeding pairs of Black-headed Gull, ten pairs of Great Crested Grebe Podiceps cristatus (L), and seven pairs of Red-headed Duck Aythya ferina (L) were identified.
The reservoir with the overgrowth of coastal aquatic vegetation was 50% characterized by the dominance of common reeds. Also attending were the groupings of the Potamogeton and the Persicaria amphibia. The breeding species were: Western Marsh Harrier Circus aeruginosus (1 pair), Purple Heron Ardea purpurea (L) (1 pair), Eurasian bittern Botaurus stellaris (L) (4 pairs), Little bittern Ixobrychus minutus (L), Water rail Rallus aquaticus (L) (2-3 pairs ) and Little Crake Porzana parva (Scopoli) (Table 1). Also, there are two species of colonial birds on this reservoir, including the Whiskered Tern, which built nests in thickets of amphibian mustard, and the Black-headed Gull, which nested on the convolutions of reeds.
On a reservoir with 90% coverage with aquatic plants, groups of common reed dominated mainly with a small number of broadleaf cattail. At the same time, a smaller number of different bird species were found and a smaller number of nests.
We registered that the maximum and minimum percentage of overgrowth of water bodies negatively affects the reproduction of most waterbirds. On reservoirs with overgrowth in 5% and 90%, nesting of 8 and 6 species were found, respectively. The species diversity of these reservoirs is also insignificant.
According to the above research results, it follows that in a reservoir with 23% overgrowth, the quantitative composition of the Great Crested Grebe (12 pairs) and the Pochard (7 pairs) was the highest. Such conditions can be considered favorable for the reproduction of these species, in particular, due to the presence of open areas with a sufficient overgrowth area, which is necessary for nesting. Simultaneously, when the reservoir is overgrown with macrophytes by more than 50%, the number of pochard nesting pairs decreases, but under such conditions, the number of nesting pairs of Eurasian bittern and Great White Egret that is most likely due to the optimal percentage of water plants that represent additional bird stations. The ratio of the sizes and types of overgrowth: islets, the formation of bays on water bodies, determining factors in the distribution and nesting of species (Fig. 3).

Fig 3: Fragment of a nesting colony of Whiskered Tern on thickets of Persicaria amphibia
The basis of overgrowing of the reservoir was 33% common reed with significant formations of broadleaf cattail and narrow-leaved cattail. It should be noted that there was a colony of 6 pairs of Black tern Chlidonias nigra (L) and 15 pairs of Black-Headed Gull. The nests of gulls were arranged on hummocks and terns on the folds of cattail.
In a reservoir with an area of overgrowth of 23%, the dominant plant groups were common reed and broadleaf cattail. A colony of 50 pairs of Black-headed gull was present in the reservoir (Fig. 4). Two nests of Grey Heron Ardea cinerea (L) and four pairs of Great Crested Grebe were found.

Fig 4: Colony of Black-headed Gull in the thickets of reed
To determine the dominant bird species in the studied water bodies, the dependences of the qualitative composition of the avifauna with the composition of nesting pairs were analyzed, and the number depended on the degree of overgrowth of the water body. During the overgrowth of water bodies, we found that 50% of the dominant breeding species were Black-heated Gull, Cool, and Whiskered Tern. With overgrowth of 23%, the dominant species were the l, Whiskered Tern, and Black-necked Grebe Podiceps nigricollis (C.L.Brehm), while with overgrowth of 33%, the dominant species were: Black-heated Gul and Cool (Fig. 5, 6)
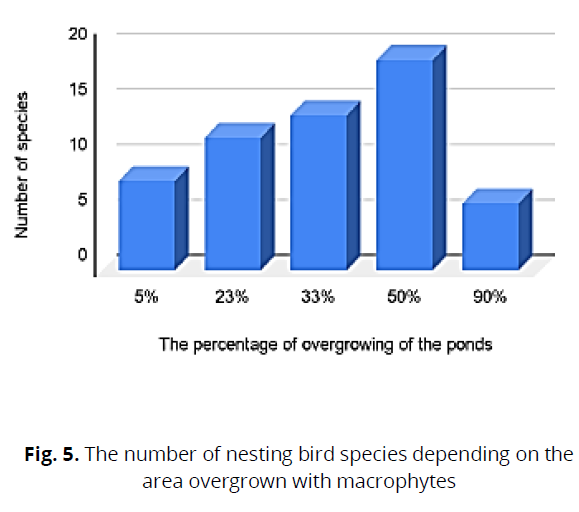
Fig 5: The number of nesting bird species depending on the area overgrown with macrophytes
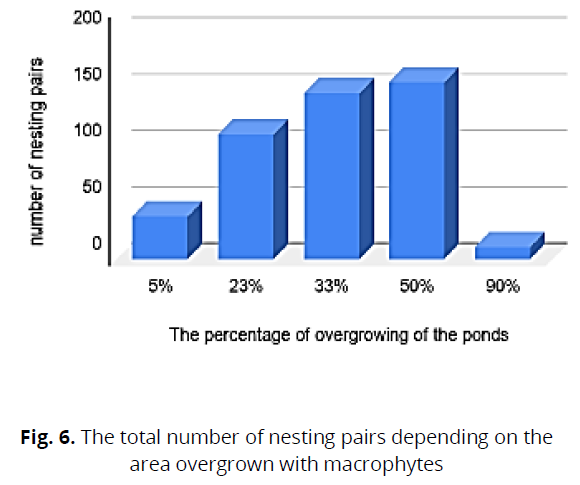
Fig 6: The total number of nesting pairs depending on the area overgrown with macrophytes
Significant areas of overgrowth (by 90%) with air-water plants, especially reed, radically reduce the number and species composition of nesting birds. A large number of passerines nest on reservoirs of this type (Great Reed Warbler Acrocephalus arundinaceus (L), Reed Warbler Acrocephalus scirpaceus (Hermann), March Warbler Acrocephalus palustris (Bechstein), Bearded Tit Panurus biarmicus (L), Common Reed Bunting Emberiza schoeniclus (L)).
The close relationship between the percentage of overgrowth of the reservoir and plants is confirmed by statistical calculations (Table 2). It should be noted that the highest percentage of water bodies overgrowth was not taken into account for calculations.
| Factors | ||||
|---|---|---|---|---|
| r | rs | t | R2 | p-value |
| 0.94 | 1 | 3.96 | 0.88 | <0.05 |
Table 2. Results of statistical calculations of the relationship between the percentage of overgrowth of the reservoir nesting birds (from 5 to 50%)
The results obtained are statistically significant and indicate a close correlation between the degree of overgrowth of water bodies with macrophytes and nesting pairs of birds. The average error of the results obtained, which characterizes the adequacy of the regression model, is 22.7%. Based on this, it can be argued that the nesting of some species of water-marsh birds depends on the degree of overgrowth of reservoirs with higher aquatic plants. Thus, the most optimal conditions for the existence and reproduction of waterfowl and water-marsh birds were formed when water bodies were overgrown by 33% and 50%. The high diversity of these reservoirs is confirmed by the Shannon index, which is 2.04 and 2.4, respectively (Fig. 7). The species diversity of birds probably depends on the diversity of the habitat, since with a large and small percentage of overgrowth of water bodies (by 5% and 90%, respectively), in both cases, it was 1.58.
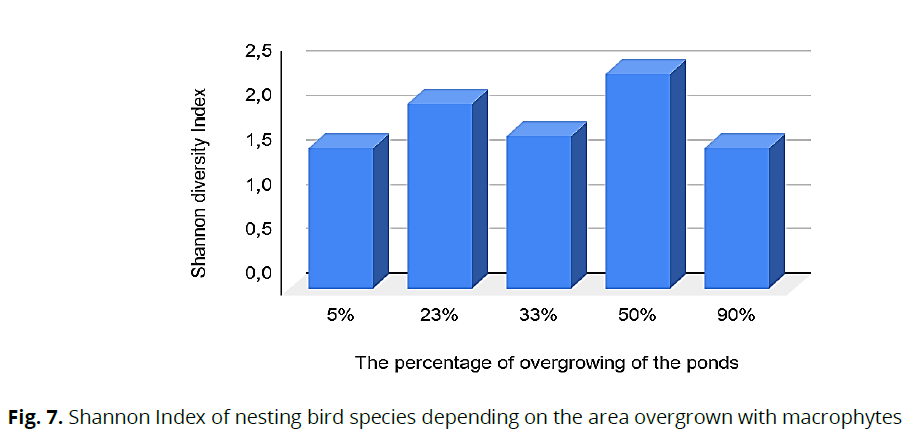
Fig 7: Shannon Index of nesting bird species depending on the area overgrown with macrophytes
Our studies were based on reservoirs with the most optimal environmental conditions, which are rarely preserved in cities due to anthropogenic pressure. At the same time, we will give an example of various interconnections in urban water bodies. When creating a city beach, considerable destruction of macrophyte groups, particularly common reeds, occurs, leading to a decrease in the number of fish spawning grounds and bird nesting grounds. If in 2015-2018 there were 12-16 nests of soaring coots on the reservoir and 10-15 nests of warblers sp., Then after the transformation of the environment with the arrangement of the recreational zone in 2019, this number decreased to 3 and 2 pairs, respectively (Kovalenko & Prychepa, 2021). This once again confirms the leading role of higher aquatic plants in the preservation of biodiversity. Сertain species of living things can adapt to toxicants and noise pollution, but only in the presence of habitat diversity.
In the future, the results can be used when planning technical work on sites near park reservoirs within the city, which is necessary to preserve the normal functioning of freshwater ecosystems with sustainable conservation of biodiversity and a high level of ecological well-being in cities (Thomaz & da Cunha, 2020). It is necessary to periodically regulate the groupings of higher aquatic plants (Sebastián-González et al., 2010). The reservoirs with the highest diversity had the most extended trophic connections with high nutrition and productivity of macrophytes (Declerck et al., 2011). Thus, the results obtained make it possible to predict potential changes in the structure of bird communities, depending on the development of succession. According to the research results, four everytope bird species can be distinguished, which do not require special conditions and reproduce successfully regardless of the reservoir's overgrowth. At the same time, such species as Coot, Mallard Anas platyrhynchos (L), Common Moorhen, and Little Bittern.
At the same time, such species as Pochard, Marsh Harrier, Garganey Anas querquedula (L), Little Grebs Podiceps ruficollis (Pallas), Black-necked Grebe, Little Grebe, Black-necked Grebe, Yellow-legged Gull Larus cachinnans (Pallas), Eurasian bittern, Whiskered Tern, Black Tern - require specific conditions, because an insufficient number of higher aquatic plants affects the fluctuations in nesting birds number. Therefore, they can be the indicators of this ecosystem.
Conclusions
The research results showed a close relationship between the percentage of the area overgrown with higher aquatic plants and the nesting of waterbirds. We found that the highest number of bird species (156 pairs) nest in reservoirs overgrown with higher aquatic plants by 50%. When water bodies are overgrown with macrophytes by 33%, some species spread, and other species decrease.
When water bodies are overgrown with macrophytes by 23%, there is a smaller number of species and nesting pairs of birds than water bodies overgrown by 33 and 50%. Fragmentation of biotopes with overgrowth of water bodies by 5% does not provide optimal conditions for the existence and reproduction of waterbirds. The overgrowth of the reservoir by 90% significantly changes the bird community structure because some bird species disappear under such conditions.
The experience gained in the passage of natural processes in the most optimal conditions can be transferred to the water bodies of the city, which need to optimize the ecological balance due to irrational nature management.
References
Aleksanov, V.V. (2017). Metody izucheniya biologicheskogo raznoobraziya. Kaluga (in Russian).
Banik, M.V., & Dzhamirzoyev, G.S. (2004). K metodike ucheta vodoplavayushchikh ptits po vyvodkam na krupnykh vodoyemakh. Oblіk ptakhіv: pіdkhody, metodyky, rezul'taty (Zb. nauk. statey drugoi mіzhnar. nauk.-prakt. konf.), Zhytomyr, 31–34 (in Russian).
Bashchenko, M.I., Boiko, О.V., Honchar, О.F., Gutyj, B.V., Lesyk, Y.V., Ostapyuk, A.Y., Kovalchuk, І.І., & Leskiv, Kh.Ya. (2020). The effect of milk thistle, metiphen, and silimevit on the protein-synthesizing function of the liver of laying hens in experimental chronic cadmium toxicosis. Ukrainian Journal of Ecology, 10(6), 164–168. doi: 10.15421/2020_276
Bibbi, K., Dzhons, M., & Marsden, S. (2000). Metody polevykh ekspeditsionnykh issledovaniy. Issledovaniya i uchety ptits. Konsul'tativnyy tsentr ekspeditsiy. Moscow (in Russian).
Bochenski, Z. (1995). The effect of fishponds on the regional bird fauna. Acta Hydrobiol, 37(11), 75–82.
Borowiec, M., Stawarczyk, T., & Witkowski, J. (1981). Próba uściślenia metod oceny liczebności ptaków wodnych. Not. Orn., 22(1-2), 47–61.
Borshch, O.O., Borshch, O.V., Sobolev, O.I., Nadtochii, V.M., Slusar, M.V., Gutyj, B.V., Polishchuk, S.A., Malina, V.V., Korol, A.P., Korol-Bezpala, L.P., Bezpalyi, I.F., & Cherniavskyi, O.O. (2021). Wind speed in easily assembled premises with different design constructions for side curtains in winter. Ukrainian Journal of Ecology, 11 (1), 325–328. doi: 10.15421/2021_49
Borshch, O.O., Gutyj, B.V., Borshch, O.V., Sobolev, O.I., Chernyuk, S.V., Rudenko, O.P., Kalyn, B.M., Lytvyn, N.A., Savchuk, L.B., Kit, L.P., Nahirniak, T.B., Kropyvka, S.I., & Pundyak, T.O. (2020). Environmental pollution caused by the manure storage. Ukrainian Journal of Ecology, 10(3), 110–114. doi: 10.15421/2020_142
Breeding Bird Atlas of Europe (1992). Working Report 1: Non-passeriformes. The Netherlands, 257.
Butsiak, H.A., Butsiak, V.I., Gutyj, B.V., Kalyn, B.M., Muzyka, L.I., Stadnytska, O.I., Luchyn, I.S., Rozputnii, O.I., Kachan, L.M., Melnichenko, Yu.O., Sliusarenko, S.V., Bilkevich, V.V., & Leskiv, K.Y. (2021). Migration of mobile forms of heavy metals into the vegetative mass of plants under local human-caused load. Ukrainian Journal of Ecology, 11 (1), 239-343. doi: 10.15421/2021_50
Declerck, S.A.J.,Bakker, E.S., van Lith,B. et al, (2011). Effects of nutrient additions and macrophyte composition on invertebrate community assembly and diversity in experimental ponds. Basic Appl. Ecol., 12(5), 466–475. doi: 10.1016/j.baae.2011.05.001.
Dombrowski, A. (1987). Badania awifauny lęgowej stawów rybnych (instrukcja). Fauna niziny Mazowieckiej. Wyższa szkoła Rolniczo-Pedagogiczna im. G. Dymitrowa w Siedlicach, 24.
Fesenko, H.V., & Bokotey A.A. (2002). Ptachy fauny Ukrainy: polʹovyy vyznachnyk. Kyiv, Ukrayinsʹke tovarystvo okhorony ptakhiv (in Ukrainian).
Honcharova, O.V., Paraniak, R.P., Kutishchev, P.S., Paraniak, N.M., Hradovych, N.I., Matsuska, O.V., Rudenko, O.P., Lytvyn, N.A., Gutyj, B.V., Maksishko, L.M. (2021). The influence of environmental factors on fish productivity in small reservoirs and transformed waters. Ukrainian Journal of Ecology, 11 (1), 176-180. doi: 10.15421/2021_27
Inventory Methods for Waterfowl and Allied Species: Loons, Grebes, Swans, Geese, Ducks, American Coot and Sandhill Crane (1999). Standards for Components of British Columbia's Biodiversity No. 18. – Resources Inventory Committee of the Province of British Columbia, 90.
Kalaitan, T.V., Stybel, V.V., Gutyj, B.V., Hrymak, O.Ya., Kushnir, L.P., Yaroshevych, N.B., Vovk, M.V., & Kindrat, O.V. (2021). Ecotourism and sustainable development. Prospects for Ukraine. Ukrainian Journal of Ecology, 11(1), 373-383. doi: 10.15421/2021_55
Kalyn, B.M., Khromova, M.V., Vishchur, V.Ia., Butsiak, H.A., Kropyvka, S.I., & Gutyj, B.V. (2020). Estimation of quality of surface water of Dniester river basin within Lviv and Khmelnytsk regions. Ukrainian Journal of Ecology, 10(6), 127-132. doi: 10.15421/2020_271
Kawatsu, M., Morimoto, G., & Kagami, M. (2015). Seasonal changes in the water bird community in Lake Inba: Influence of floating-leaved macrophytes on habitat selection. Aquatic Botany, 126, 32–37.doi: 10.1016/j.aquabot.2015.06.003.
Kazannyk, V.V., Turchyk, A.V., & Yanenko, V.O. (2014). Vodno-bolotyana ornitofauna Svyatoshynsʹkykh stavkiv m. Kyeva ta yiyi sezonni zminy. Visnyk Dnipropetrovsʹkoho ahro-ekonomichnoho universytetu, 1(33), 170–174 (in Ukrainian).
Klaassen, M., & Nolet, B.A. (2007). The role of herbivorous water birds in aquatic systems through interactions with aquatic macrophytes, with special reference to the Bewick’s Swan, Nolet Hydrobiologia, 584, 205–213. doi: 10.1007/s10750-007-0598-5.
Kofonov, K., Potrokhov, О., Hrynevych, N., Zinkovskyi, O., Khomiak, O., Dunaievska, O., Rud, O., Kutsocon, L., Chemerys, V., Gutyj, B., Fijalovych, L., Vavrysevych, J., Todoriuk, V., Leskiv, K., Husar, P., & Khumynets, P. (2020). Changes in the biochemical status of common carp juveniles (Cyprinus carpio L.) exposed to ammonium chloride and potassium phosphate. Ukrainian Journal of Ecology, 10(4), 137–147. doi: 10.15421/2020_181
Kovalenko, Yu., & Prychepa, M.V. (2021). Vydovyy sklad vodoplavnykh ta navkolovodnykh ptakhiv okremykh ozer Kyieva yak indykatory zahalʹnoho stanu navkolyshnʹoho seredovyshcha, Ekolohichni nauky, 34 (in Ukrainian).
Prysiazhniuk, N., Grynevych, N., Slobodeniuk, O., Kuzmenko, O., Tarasenko, L., Bevz, O., Khomiak, O., Horchanok, A., Gutyj, B., Kulyaba, O., Sachuk, R., Boiko, O., & Magrelo, N. (2019). Monitoring of morphological parameters of Cyprinidae liver. Ukrainian Journal of Ecology, 2019, 9(3), 162–167.
Rudenko, O.P., Paranjak, R.P., Kovalchuk, N.A., Kit, L.P., Hradovych, N.I., Gutyj, B.V., Kalyn, B.M., Sukhorska, O.P., Butsiak, A.A., Kropyvka, S.I., Petruniv, V.V., & Kovalska, L.M. (2019). Influence of seasonal factors on carp fish immune reactivity. Ukrainian Journal of Ecology, 2019, 9(3), 168–173.
Saranchuk, I.I., Vishchur, V.Ya., Gutyj, B.V., & Klim, O.Ya. (2021). Effect of various amounts of sunflower oil in feed additives on breast tissues' functional condition, reproductivity, and productivity of honey bees. Ukrainian Journal of Ecology, 11 (1), 344-349. doi: 10.15421/2021_51
Sebastián-González, E, Sánchez-Zapata, J.A., & Botella, F. (2010). Agricultural ponds as alternative habitat for waterbirds: spatial and temporal patterns of abundance and management strategies. Eur. J. Wildl. Res., 56(1), 11–20. doi: 10.1007/s10344-009-0288.
Stefanidis, K., Sarika, M., & Eva Papastegiadou, E. (2019). Exploring environmental predictors of aquatic macrophytes in water‐dependent Natura 2000 sites of high conservation value: Results from a long‐term study of macrophytes in Greek lakes. Aquatic conservation. Marine and freshwater ecosystems, 29(7), 133–1142. doi: 10.1002/aqc.3036.
Svalov, N.N. (1977). Variatsionnaya statistika. Moskva, Lesnaya promíshlennost' (in Russian).
Svitok, M., Hrivnák, R., Kochjarová, J. et al. (2016). Environmental thresholds and predictors of macrophyte species richness in aquatic habitats in central Europe. Folia Geobotanica, 51, 227–238.doi: 10.1007/s12224‐015‐9211‐2.
Thomaz, S.M., & da Cunha, E.R. (2020). The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages' composition and biodi. Acta Limnologica Brasiliensia, 22(2), 218–236 doi: 10.4322/actalb.02202011.
Zhang, Y., Jeppesen, E., Liu, X., et al. (2017). Global loss of aquatic vegetation in lakes. Earth‐Sciences Reviews, 173, 259–265.doi: 10.1016/j.earscirev.2017.08.013
Author Info
N. Hrynevych1, M. Prychepa2*, Yu. Kovalenko2, O. Vodianitskyi2, M. Svitelskyi3, O. Fotin4, L. Zahorui1, V. Zharchynska1, B. Gutyj5*, S. Kulish6, V. Honcharenko3, T. Velesyk7, R. Sachuk7, Ya. Stravsky8 and N. Boltyk92Institute of hydrobiology NAS of Ukraine, Kyiv, Ukraine
3Pollisia National University, Zhytomyr, Ukraine
4Sumy National Agrarian University, Sumy, Ukraine
5Stepan Gzhytskyi National University of Veterinary Medicine and Biotechnologies, Lviv, Ukraine
6Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
7Rivne State University for the Humanities, Rivne, Ukraine
8I. Gorbachevsky Ternopil National Medical University, Ternopil, Ukraine
9Ternopil Research Station, Institute of Veterinary Medicine NAAN, Ternopil, Ukraine
Citation: Hrynevych, N., Prychepa, M., Kovalenko, Yu., Vodianitskyi, O., Svitelskyi, M., Fotin, O., Zahorui, L., Zharchynska, V., Gutyj, B., Kulish, S., Honcharenko, V., Velesyk, T., Sachuk, R., Stravsky, Ya., Boltyk, N. (2021). The role of macrophytes in waterfowl reproduction. Ukrainian Journal of Ecology, 11 (2), 320-326.
Received: 16-Mar-2021 Accepted: 27-Apr-2021 Published: 30-Apr-2021, DOI: 10.15421/2021_117
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
