Research Article - (2022) Volume 12, Issue 2
The effect of salinity on accumulation proline, glycine betaine and mineral elements in four varieties of tomatoes
K.M. Derkaoui1*, M. Sahnoune2 and M. Belkohodja1Abstract
Salinity of soil and water, is one of the major abiotic factors limiting plant productivity and crop yields. Interest in morphological and biochemical characteristics of adaptation to environmental stresses cultivated plants has attracted the attention of many researchers. In this interest and in order to compare the salt sensitivity of tomatoes, we have carried out analyzes are carried out on four varieties of tomatoes (Marmande, Aicha, Heinz1573, Cerise), subjected to different concentration (0 mM, 70 mM, 145 mM to 200 mM). Our results show that the salt stress in a depressive effect on the growth of the tomatoes, and the four genotypes have different reactions toward the increasing doses of NaCl. The synthesis of proline reaches an average rate of the order of 0.7563 mg/ml at 200 mM with Marmande variety. At the aerial level, glycine betaine has a beneficial effect on the achievement of osmotic adjustment which has reached a high content of the order of 1.79 mg/ml at 200 mM of Nacl in the leaves of tomatoes. In addition, salt stress affected the mineral composition by increasing the leaf content of Na+, but the rate of K+ and Ca+2 decreased significantly K+ until 173, 72 mg/g PSF and Ca+2 until 62 mg/g PSF respectively from control to 200 mM.
Keywords
Glycine betaine, Mineral composition, Leaf dry weight, Proline, Salinity and Tomato.
Introduction
Salinization is the major process of land degradation. It is one of the abiotic factors the most widespread in the world and which greatly reduces agricultural yields. The harmful effect of this constraint is generally manifested by the limitation of the productivity of cultivated plants (Ashraf 1999). This is because salinity affects several aspects of plant physiology, growth and development (Borsani, et al., 2003). A metabolic response to salt stress is the synthesis of compatible osmolytes. These organic compounds play a role in combating environmental stresses in living organisms. In many cases, environmental stresses threaten the stability of protein conformation and therefore various osmolytes have been selected to stabilize intracellular macromolecules (Hong, et al., 1992; Smirnoff 1993) response to salinity, therefore, genetic variability within a species is a valuable tool for screening and selecting the most salt-tolerant varieties (Foolad, 1996; Cuartero, et al., 1999). Plants faced with undesirable situations such as high salt concentrations decrease their osmotic potential by accumulating osmolytes that do not disrupt enzyme functionsso as to maintain water uptake in water-poor soil (Demiral and Turkan 2006). The accumulation of these compatible solutes (osmoprotectants) such as proline and glycine betaine, allows the maintenance of turgor and stabilization of proteins and membranes against the destabilizing effects of abiotic stresses including salinity, drought and high temperature, which cause the depletion of cellular water. Therefore, the accumulation of these solutes is an important factor that helps the plant to adapt to the saline environment (Bohnert, et al., 1995; Garcia, et al., 1997). The physiological significance and mechanisms leading to proline accumulation in the genus Lycopersicum are poorly understood. Furthermore, according to Cram (1976), sugars contribute up to at 50% of the total osmotic potential in glycophytes subjected to saline conditions. The survival of tomato plants in saline soils depends on their ability to synthesize organic compounds, such as proline and sugars (Fariba and Ehsanpour 2005; Flowers 2004) and the accumulation of Na+ and K+ (Borsani, et al., 2003) to reduce the damage of salt stress. Tomato cultivars can differ in their susceptibility to salt stress (Alian, et al., 2000; Chookhampaeng, et al., 2007), hence selection of tolerant cultivars can help improve the performance of tomato plants under saline conditions. Potassium is an essential nutrient being the main inorganic nutrient for many land plants. As a result, the K+ transport systems implying a good selectivity of K+ on Na+ can also be considered as an important factor of salinity tolerance (Rodriguez-Navarro 2000). In general, most research on salt tolerance in tomato has been developed in the wild vs. domesticated species (Kahlaoui, et al., 2013). And very few reports on commercial cultivars are available. The main objective of the present work was to evaluate the effects of salt stress on tomato (var. microtom) plant biochemistry and tolerance threshold. More detailed study in this area is needed, including examining the effect of NaCl on compatible solutes like proline, glycine betaine, minerals like Na+, K+, Mg2+ and Cl- in tomato.
Materials and Methods
Preparation of the plant
The sowing of tomato seeds was carried out in the nursery and then the transplanting of the seedlings was carried out in a pot in a greenhouse Experimental protocol Location of the test The trial was conducted at the Faculty of Natural and Life Sciences in Tiaret; under semi controlled conditions (plastic greenhouse). The plant material used The varieties of tomatoes tested in this test are : Marmande, Heinz 1573 Cerise and Aicha. Experimental device The test shall be conducted in plastic cylinders (P.V.C) with a diameter of 10 cm and a height of 100 cm, each filled with a substrate composed of a mixture of well-decomposed organic matter, sand and earth according to the respective proportions of 1.8.1. This device has three blocks; each block consists of 12 cylinders with four varieties of tomato repeated with three repetitions. three saline concentrations are applied with 75, 145 and 200 mM of NaCl respectively. For the control lot is irrigated with tap water (not salted). The experiment was conducted according to a random experimental device with four treatments. Sowing is carried out in the nursery on March, 2013 at the rate of two seeds per pot at a depth of 1 cm. Transplanting is carried out one month after sowing at the rate of one plant per cylinder. The seedlings are watered with a nutrient solution until the six-leaf stage when saline treatment has begun. Phytosanitary treatment Before the start of the experiment, the substrate used for the experiment was treated with an insecticide (karate) and a fungicide (Anvil5Sc). Irrigation Every 48 hours’ irrigation is maintained at capacity in the field by the contribution of 250 ml of water for all lots; to calculate the watering dose, the weight of the pot is subtracted after twenty-four hours of wiping from the weight of the pot saturated with water. From transplanting until the application of saline stress, irrigation is provided with a nutrient solution, diluted in distilled water at a rate of 2 g/l. This solution contains balanced nutrients to strengthen the vigor of plants, stimulate rooting, improve the quality of crops and help the plant recover quickly in the event of a climatic accident. Methods and measurements carried out The measurements were carried out one month after the application of the saline solution and concerned the morphological and physiological parameters of the aerial part.
Proline determination
To 20 mg of the dry weight of the sample was added 1 ml of sulfosalicylic acid (3%). The resulting solution was stirred for 1 hour at 30°C. The samples were then centrifuged at 10000rpm for 5 minutes at 4°C. 125μl of the supernatant of each sample was put in test tubes to which 125μl of glacial acid and 125μl of hydronin acid were added respectively. The tubes are then incubated in a water bath for 1 hour at 90°C. The samples were cooled by immersing them in ice for 1 minute (Bates et al. 1973). Finally, 4 ml of toluene was added to each tube and the reading was determined at 520 nm.
Determination of glycine betaine
To 0.5 g of the finely ground dry plant material was added 20 ml of distilled water. The solution obtained was incubated for 48 h at 25°C. The samples are then filtered and the filtrate is stored in the freezer until analysis. The thawed extracts were diluted 1:1 with 2 N sulfuric acid. 0.5 ml of the resulting solution was cooled in ice water for 1 h. KI-I2 reagent (0.2 ml) was added and the mixture was gently vortexed. The samples were stored at a temperature of the order of 0-4°C for 16 hours. Then, the samples were transferred into tubes to centrifuge them at 10,000 g for 15 minutes at 0°C. The supernatant is aspirated carefully with a micropipette. As the solubility of the complex increases markedly with temperature, it is important that the tubes be kept cold until the complex is separated from the acid medium. The pellet is dissolved in 9 ml of 1,2-dichloroethane (reagent). First wash with 0.5 ml and leave for 5 minutes. Mix the tubes with the vortex to achieve complete solubility in the solvent. After 2 hours, the absorbance was measured at 365 nm with a UV- spectrophotometer, visible (Gieve and Grattan 1983). Glycine betaine standards (50-200 pg/ml) were prepared in 2 N sulfuric acid and the procedure for estimating the sample was followed.
Determination of mineral matter composition
The plant was harvested after the treatments as defined and separated from the roots, and dried in a forced air oven at 65°C for 4 days for the determination of the dry weight (DS). The dried material was digested with HNO3:HClO4 (2:1, v:v). Concentrations of K+, Mg2+ and Na+, Cl- weredetermined by ICP-MS using an Iris Intrepid ICP spectrometer (Thermo Electron Corporation, Franklin, USA). Data reported are means of 3 values per treatment and error bars represent standard error.
Statistical analysis
Statistical processing was performed by Excel, Version 2016 and the parameters recorded were subjected to two-way analysis of variance. Comparison means were performed by ANOVA test at the significant level of 0.05.
Results and Discussion
Effect of salt stress on leaf dry weight
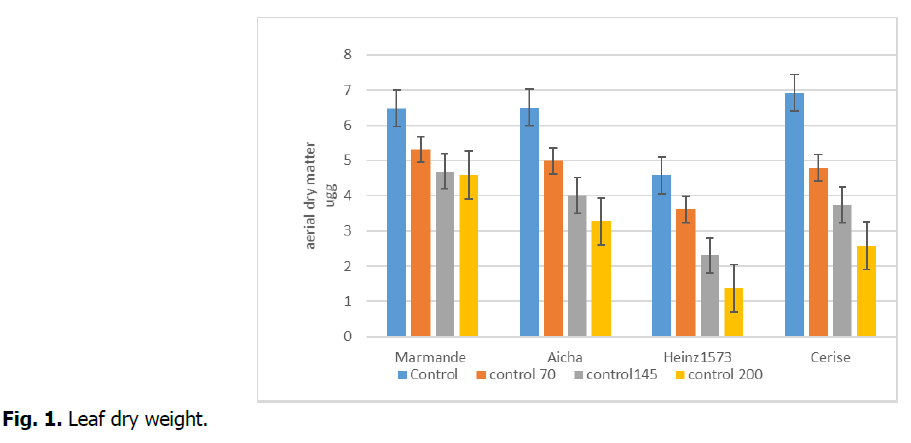
Increasing Na+ in the solution significantly decreased leaf growth of tomato. Salinity had a more negative impact on the production of the dry biomass of the aerial part of the plants of this glycophyte. The maximum production (5.31 g) of dry matter was observed at an average salt concentration of 70 mM (Fig. 1). Attenburrow and Waller (1980) observed a significant drop in dry matter yield of tomato plants by increasing salinity. Similarly, this is observed in our work, compared to the 1 mM control treatment, a remarkable decrease in dry matter production was observed in tomato plants. Variations in dry matter production were also dependent on salt types (Ryan, et al., 1975) and among the soluble salts NaCl is the most detrimental to plant growth and nutrient uptake (AL-Rawahy, et al., 1990). In their experiments, seawater containing high amounts of NaCl produced a reduced amount of dry matter compared with soil salinity. Salinity decreases leaf area and relative growth rate (Alarcon, et al., 1994) which may contribute to reduced weight and nut production in tomato (Mahajan and Tuteja 2005).
Fig 1. Leaf dry weight.
The impact of this abiotic constraint on dry aboveground biomass was also reported by Laaziza, et al., (2003), salinity reduced the growth of the aerial parts of clover more than that of the roots. Similar results were reported by Dubey and Singh (1999).
Effect of salt stress on proline
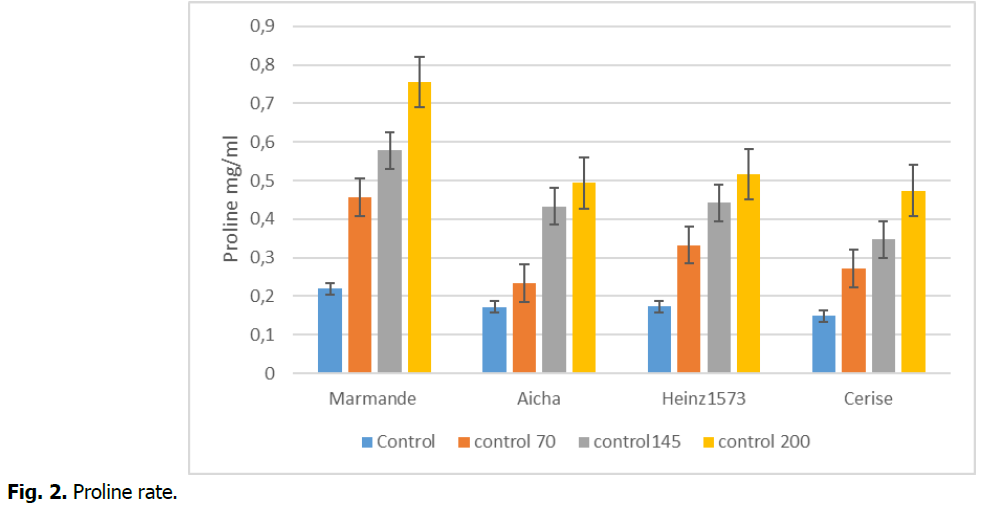
The results of more recent authors are compatible with our current work, proportionally with the progression of salt stress, proline synthesis increases gradually and more precisely in tomato leaves. The highest concentration of proline is marked in the stressed marmande variety (200 mM) with a maximum value of 0.7563 (mg/ml) compared to the other concentrations, on the other hand the stressed cherry variety marked a minimum value of 0.474 (mg/ml) compared to the other concentrations (Fig. 2). These data are confirmed by the anova test which is very significant p<0.05.
Fig 2. Proline rate.
The accumulation of proline is one of the most remarkable manifestations in plants to limit the effects of salt and water stress in order to achieve the adjustment of the osmotic potential in the cytoplasm (Sannada, et al., 1995 ; Belkhodja and Benkablia, 2000).
Our results are consistent with those found by Syed, et al., (2011) on tomato, which suggests that the ability to increase proline synthesis under salt stress can determine plant tolerance levels to salinity. It is possible that enhanced proline accumulation may regulate several processes necessary for survival in the salt stress condition (Maggio, et al., 2002).
Effect of salt stress on glycine betaine
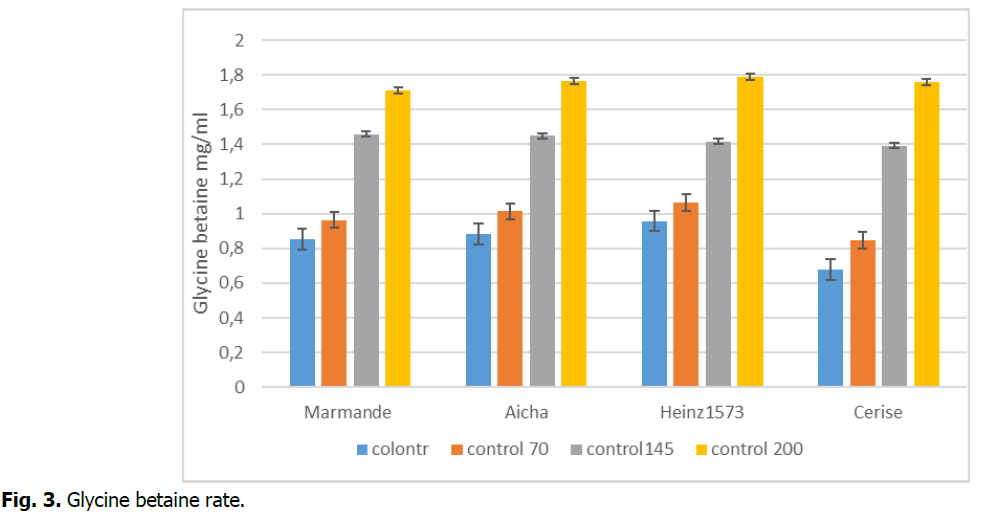
The statistical study was very significant p<0.05, because variations in the magnitude of this parameter were howevernoted (Fig. 3). Our study shows that the variety heinz1573 recorded a significant accumulation of glycine betaine for the highest concentration (200 mM), with a maximum value of 1.79 (mg/ml) compared to the other concentrations, on the other hand the stressed variety marmande and for the concentration (200 mM) marked a minimum value of 1.71 (mg/ml) compared to the other concentrations. Other work has studied the synthesis of glycine betaine in various plants subjected to salt stress. In the case of osmoregulation, the compatibility of this glycine betaine solute (Glybet) is a compound that can potentially play a crucial role in effective protection against salt, drought, and extreme temperature stress (Achraf and Harris 2004; Achraf and Foolad 2007; Chen and Murata 2008).
Fig 3. Glycine betaine rate.
Effect of salt stress on mineral composition in the aerial part
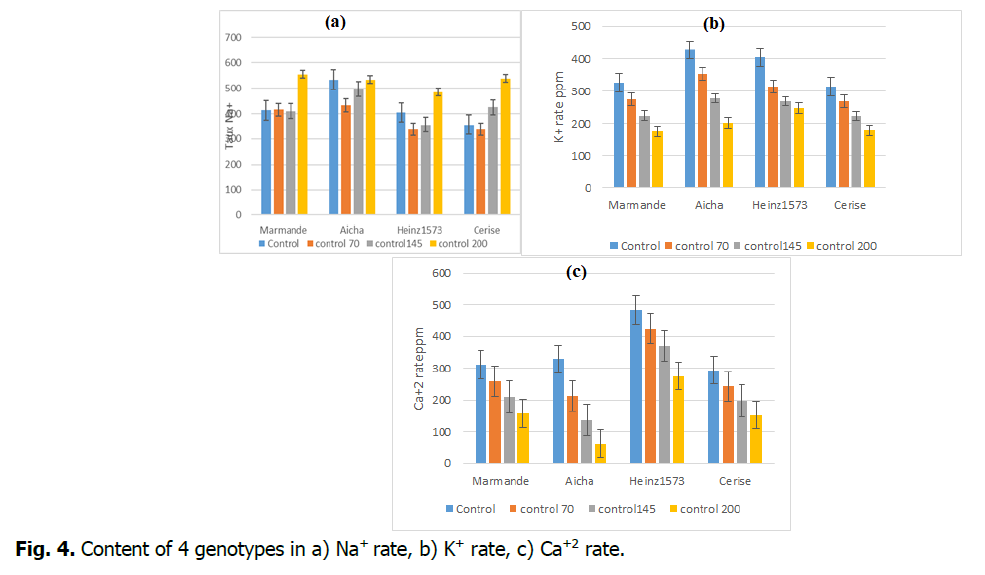
Salt tolerance in higher plants depends on how plants control salt transport through organs (Niu, et al., 2010). Indeed, salt tolerance mechanisms are of three distinct types in plants: those that tolerate osmotic stress, those that exclude Na+ and Cl- from their tissues and those that tolerate the accumulation of Na+ and Cl- in their tissues. their tissues (Haouala 2007, Munns and tester, 2008). This sensitivity or tolerance to salinity varies between species and varieties. In this study, the high sensitivity of the marmande variety to salinity indicates that it does not tolerate a high accumulation of Na+ in its tissues. Indeed, it is the variety most affected by salt. The most tolerant variety is Heinz 1573, it has the lowest Na+ content, which indicates that it is of type “exclude”. It develops mechanisms to limit the accumulation of Na+ in its tissues (Maeshner, 1995).
Aicha and cherry hold an intermediate place between Marmande and Heinz1573, this indicates that these cultivars have some sodium tolerance but not as high as Marmande. Salinity can affect Ca2+ and K+ uptake, depending on species and salinity level (Grattan and Grieve, 1999) (Fig. 4).
Fig 4. Content of 4 genotypes in a) Na+ rate, b) K+ rate, c) Ca+2 rate.
The effect of salt stress on Ca2+ and K+ content varied with varieties and salt load of irrigation water. We noticed two kinds of behavior which can be summarized as follows:
• Statistical analysis showed significant difference for Ca2+ and K+ contents in all varieties regardless of the salt treatment applied.
• The potassium and calcium levels of the leaves decreased under the effect of the salinity mainly for the Aicha variety, the reduction in Ca2+ is the lowest value recorded, being 62 ppm for 200 mM. For the K+ ion, this fall is 173, this is the lowest value displayed by the Marmande cultivar.
Authors Contribution
Derkaoui designed and implemented the project and wrote the manuscript. Pr Sahnoune contributed each to the paper write-up at different level; Pr Belkhodja supervised the project. All authors discussed the results and contributed to the final manuscript.
Acknowledgment
The authors express their gratitude to Pr. Ahmed Adda, director of the vegetal biology Laboratory, biology and agriculture institute ‘s staff of Tiaret for the reception and hospitality. They also want to thank the beekeepers for their collaboration.
References
Alarcon, J.J., Blanco, M.J.S., Bolarin, M.C., Torrecillas, A. (1994). Growth and osmotic adjustment of twotomato cultivars during and after saline stress. Plant and Soil, 166:43-47.
Albacete, A., Martı ́nez-Andu ́jar, C., Ghanem, M.E., Acosta, M., Sa ́nchez-Bravo, J., Asins, M.J., Cuartero, J., Lutts, S., Dodd, I.C., Pe´rez-Alfocea, F. (2009). Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ, 32:928-938.
Alian, A., Altman, A., Heuer, B. (2000). Genotypic difference in salinity and water stress tolerance of fresh market tomato cultivars. Plant Science, 152:59-65.
Al-rawahy, S.A., Stroehlein, J.L., Pessarakli, M. (1990). Effect of salt stress on dry matter production and nitrogen uptake by tomatoes. Journal of Plant Nutrition, 13:567-577.
Ashraf, M. (1999). Breeding for salinity tolerance proteins in plantsCrit. Review Plant Science, 13:17-42.
Ashraf, M., Foolad, M.R. (2007). Roles of glycine betaine and proline in improving plant abiotic stressresistance. About Experimental Botany, 59:206-216.
Ashraf, M., Athar, H.R., Harris, P.J.C., Kwon, T.R. (2008). Some prospective strategies for improving crop salttolerance. Advance Agronomy, 97:45-110.
Attenbury, D.C., Waller, E.L. (1980). Sodium chloride; its effect on nutrient uptake and crop yields withtomatoes in NFT (nutrient film technique). Acta Hortic, 98:229-236.
Bates, L., Waldren, R.P., Teare, I.D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39:205-207.
Belkhodja, M., Benkablia, M. (2000). Proline response of Faba bean under salt stress. Egypt Journal of Agriculture Research, 78:185-195.
Bohnert, H.J., Nelson, D.E., Jensen, R.G. (1995). Adaptations to environmental stresses. Plant Cell, 7:1099-1111.
Bolarin, M., Fernandez, F., Cruz, V., Cuartero, J. (1991). Salinity tolerance in four wild tomato species usingvegetative yield-salinity response curves. Journal of American Society Horticulture Science, 116:286-290.
Borsani, O., Valpuesta, V., Botella, MA. (2003). Developing salt tolerant plants in a new century: Amolecular biology approach. Plant Cell Tissue and Organ Culture, 73:101-115.
Chen, T.H.H., Murata, N. (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trend Plant Science, 13:499-505.
Chen, G., Fu, X., Lips, S.H., Sagi, M. (2003). Control of plant growth resides in the shoot, and not in the root, in reciprocal grafts of flacca and wild-type tomato (Lycopersicon esculentum), in the presence and absence of salinity stress. Plant Soil, 256:205-215.
Chookhampaeng, S., Pattanagul, W., Theerakulpisut, P. (2007). Screening some tomato commercialcultivars from Thailand for salinity tolerance. Asian Journal Plant Science, 6:788-794.
Cram, W.J. (1976). Negative feedback regulation of transport in cells. The maintenance of turgor, volume and nutrient supply. Encyclopaedia Plant Physiology, 2:284-316.
Cuartero, J., Fernandez-Munoz, R. (1998). Tomato and salinity. Scientia Horticulture, 78:83-125.
Demiral, T., Turkan, I. (2006). Exogenous glycinebetaine affects growth and proline accumulation anddelays senescence in two rice cultivars under NaCl stress. About Experimental Botany, 56:72-79.
Dubey, R.S., Singh, A.K. (1999). Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolizing enzymes in rice plants. Biological Plant, 42:233-239.
Fariba, A., Ehsanpour, A.A. (2005). Soluble proteins, proline, carbohydrates and Na+/K+ changes in twotomato (Lycopersicon esculentum Mill.) cultivars under in vitro salt Stress. American Journal of Biochemistry and Biotechnology, 1:212-216.
Flowers, T.J. (2004). Improving salt tolerance. Journal of Experimental Botany, 55:307-329.
Foolad, M.R. (1996). Genetic analysis of salt tolerance during vegetative growth in tomato, Lycopersiconesculentum Mill. Plant Breed, 115:245-250.
Francesco Di, G., Angelo, S. (2013). Grafting improves tomato salinity tolerance through sodium partitioning within the shoot. Hortscience, 48:855-862.
Garcia, A.B., Engler, J.D.A., Iyer, S., Gerats, T., Montagu, M.V., Caplan, A.B. (1997). Effects ofosmoprotectants upon NaCl stress in rice. Plant Physiology, 115:159-169.
Gieve, C.M., Grattan, S.R. (1983). Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant Soil, 70:303-307.
Grattan, S.R., Grieve, C.M. (1999). Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli, M. (Ed.), Handbook of Plant and Crop Stress. Marcel Dekker, New York, pp:203-226.
Haouala, F., Ferjani, H., Ben El Hadj, S. (2007). Effet de la salinité sur la répartition des cations (Na+, K+ et Ca2+) et du chlore (Cl-) dans les parties aériennes et les racines du ray-grass anglais et du chiendent. Bitechnology Agronomic Social Environment, 11:235-244.
Hayet, B., Reyes, R., Elvira, L.G., Manuel, F.G.L., Manuel, N.C., Rosa, M.R., Vicente, M., Francisco, R. (2015). High Ca2+ reverts the repression of high-affinity K+ uptake produced by Na+ in Solanum lycopersycum L. (var. microtom) plants. Journal of Plant Physiology, 180:72-79.
Hong, B., Barg, R., Ho, T.H. (1992). Developmental and organ specific expression of an ABA and stress induced protein in barley. Plant Molecular Biology, 18:663-674.
Juan, M., Rivero, R.M., Romero, L., Ruizet, J.M. (2005). Evaluation of some nutritional and biochemicalindicators in selecting salt-resistant tomato cultivars. Environmental and Experimental Botany, 54:193-200.
Kahlaoui, B., Hachicha, M., Teixeira, J., Misle, E., Fidalgo, F., Hanchi, B. (2013). Response of two tomato cultivars to field-applied proline and salt stress. Journal of Stress Physiology and Biochemistry, 9:357-365.
Laaziza, B.K., Asun ́cion, G., Mario, H., Abdallah, O. (2003). Effect of salt stress in hydroponicson clover inoculated by Rhizobium. Agronomy, EDP Sciences, 23:553-560.
Maggio, A., Miyazaki, S., Veronese, P., Fujita, T., Ibeas, J.I., Damsz, B., Narasiman, M.L., Hasegawa, P.M., Joly, R.J., Bressan, R.A. (2002). Does proline accumulation play an active role in stress induced growth reduction? Plant Journal, 31:699-712.
Mahajan, S., Tuteja, N. (2005). Cold, salinity and drought stresses: An Overview. Archive of Biochemistry and Biophysics, 444:139-158.
Marschner, H. (1995). Saline soils, mineral nutrition of higher plants. 2nd ed. Academic Press, San Diego, CA.
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review Plant Biology, 59:651-681.
Niu, G., Rodriguez, D.S., Starman, T. (2010). Response of bedding plants to saline water irrigation. Hort Science, 45:628-636.
Rodriguez, N.A. (2000). Potassium transport in fungi and plants. Biochemistry and Biophysics Acta, 1469:1-30.
Ryan, J., Miyamoto, S., Stroehlein, J.L. (1975). Salt and specific ion effects on germination of four grasses. Journal Range Manage, 28:61-64.
Sannada, Y., Ueda, H., Kuribayashi, K., Andoh, T., Hayashi, F., Tamai, N., Wada, K. (1995). Novel light-dark change of proline levels in halophyte (Mesembranthemumcrystallinum L.) andglycophyts (Hordeumvulasare L and Triticumaestivum L.) leaves and rootsundersalt stress. Plant Cell, 36:965-970.
Syed, G.A., Abdur, R.N., Ullah, K., Khalid, N. (2011). Enhanced proline synthesis may determineresistance to salt stress in tomato cultivars. Pakistan Journal of Botany, 43:2707-2710.
Author Info
K.M. Derkaoui1*, M. Sahnoune2 and M. Belkohodja12Department of Biology, University of Tiaret, Ibn Khaldoun, Tiaret 14000, Algeria
Citation: Derkaoui, K.M., Sahnoune, M., Belkhodja, M. (2022). The effect of salinity on accumulation proline, glycine betaine and mineral elements in four varieties of tomatoes. Ukrainian Journal of Ecology. 12:58-63.
Received: 02-Feb-2022, Manuscript No. UJE-22-53239; Accepted: 28-Feb-2022, Pre QC No. P-53239; Editor assigned: 04-Feb-2022, Pre QC No. P-53239; Reviewed: 16-Feb-2022, QC No. Q-53239; Revised: 22-Feb-2022, Manuscript No. R-53239; Published: 08-Mar-2022, DOI: 10.15421/2022_345
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.