Research Article - (2021) Volume 11, Issue 3
The current state of water quality and benthic invertebrate fauna in Chikke Stream (North-Central Nigeria)
Y.M. Mohammed1*, F.O. Arimoro1, A.V. Ayanwale1, K.M. Adamu2, U.N. Keke1, M.D. Abubakar1, A.C. Achebe<1 and A.C. Achebe<1Abstract
The Chikke Stream Bida, Northcentral Nigeria is an important water source for its riparian communities. This study evaluates the impact of habitat structure and anthropogenic activities on Chikke Stream using physicochemical parameters and macroinvertebrate assemblages collected over eight months (January-August 2017) using modified kick sampling techniques. Three sampling stations characterized by various human activities were selected across the stream. The physicochemical parameters examined were the water temperature (24.3-27.6 °C), depth (17.0-28.6 cm), flow velocity (0.301-0.408 m/s), pH (6.0-8.9), conductivity (30-136 μS/cm), alkalinity (10-26.0 mg/L), turbidity (28-152 NTU), dissolved oxygen (2.60-8.0 mg/L), and biochemical oxygen demand (3.0-5.1 mg/L); we also determined the contamination of nitrates (0.99-4.20 mg/L) and phosphates (0.37-0.85 mg/L). A total of 719 individuals from 35 species and 24 families of invertebrates were collected from the sampling stations. The CCA results revealed positive correlation between species abundances and measured environmental variables. benthic invertebrates clustered strongly by stations than by seasons, indicating that water quality differences between the stations were responsible for the observed differences in the macroinvertebratebiotic. High densities of pollution tolerant macroinvertebrate groups and the deteriorating quality of the surface water during the sampling period indicated organic pollution stress caused by different anthropogenic activities, decomposing domestic wastes, and vegetative nature of the stream. Bottom sediment, microhabitat obliteration, and poor water quality were the key factors responsible for the macroinvertebrate assemblage structure in the Chikke Stream.
Keywords
Physicochemical, Macroinvertebrates, Assemblages Anthropogenic activities, Chikke Stream.
Introduction
Macroinvertebrate organisms are a significant part of an aquatic environment and are of economic and ecological importance as they maintain various interaction levels between aquatic ecosystems (Arimoro and Keke, 2016). Knowledge of the structure of macroinvertebrate communities gives precise and local information on recent events, which can be seen in their structuring (Marques et al., 2003). Macroinvertebrates are valuable bio-indicators providing a more accurate understanding of changing aquatic conditions than physicochemical data, giving short-term fluctuations (Arimoro and Ikomi, 2008). Many freshwater resources draining urban settlements are usually contaminated through input associated with human activities (). Nigerian aquatic systems are subjected to pollution pressures associated with urbanization and population growth (Edokpayi et al., 2010; Nkwoji et al., 2010). Pollutants from land-based activities include domestic and industrial effluents, urban storms, and agricultural runoffs (Andem et al., 2014). The introduction of these pollutants into aquatic systems flowing through Nigerian cities and villages constitute a significant threat to the physical, chemical, and faunal characteristics of the aquatic environment (Edokpayi et al., 2010)
In Nigeria, most streams have been subjected to an increasing pollution load from contaminated urban runoff water originating from industrial, agricultural, residential, commercial, and recreational areas and institutions such as schools and hospitals (Emere and Nasiru, 2009).
Macroinvertebrate taxa richness varied as a function of hydrology, season, and microhabitat. Many lotic ecosystems are spatially and temporally heterogeneous, but none more so than dry land streams flowing through arid and semi-arid landscapes. The seasonal variation in richness and trait composition is critical to our fundamental understanding of these dynamic stream networks (Giam and Olden, 2016). Temporal changes in invertebrate communities can result from seasonal dry-wet cycles and associated physicochemical changes. In the dry season, habitat area and types are often reduced (from rifles and pools to drying pools), resulting in lower taxa richness or diversity in some systems (Garcia-Roger et al., 2011). In other systems, mechanisms are less straightforward. The abundance of stream invertebrates is influenced by environmental conditions such as hydraulic stress, temperature, and water chemistry (Nicola et al. 2010; Linares et al., 2013). The dry and rainy season variation is essential to determine ecological changes in the tropics; rainfall distribution patterns have a significant impact on both the chemistry of water and the population dynamics of the fauna (Linares et al., 2013). Changes in characteristics, habitat and environmental resources of rivers can strongly influence spatial sand temporal distribution patterns in benthic communities; abiotic environmental conditions greatly influence the structure and organization of the aquatic insect communities, biotic conditions, and dispersal processes (Wibowo and Santoso, 2017).
Freshwater ecosystems are increasingly being studied worldwide because of their role in conserving and sustaining several global importance species (Arimoro et al., 2015; Edegbene et al., 2015). Freshwater pollution by human activities is becoming a matter of urgent concern threatening environmental productivity, sustainability, and further social-economic development in Africa (Nyenje et al., 2010; Arimoro and Keke, 2016). Several uses of aquatic ecosystems include water source for drinking, laundry, irrigation, hydropower generation as well as riparian activities on rivers catchments such as unregulated land use and landscape alteration, have led to both biotic and physical deterioration of the aquatic environment (Nyenje et al., 2010; Kun Li et al., 2015). Temporal variation in the benthic community is driven mainly by changes in temperature, which affects salinity, dissolved oxygen, and pH of seawater (Amini-Yekta et al., 2013). The spatiotemporal, functional, and structural compositions of macroinvertebrate assemblage in any stream. aquatic ecosystem can be influenced by anthropogenic activities (Arimoro et al., 2012; Zajac et al., 2013). In the presence of changing and intensifying human activity in catchments draining into the stream, there is a need to evaluate the current status of water quality and benthic invertebrate fauna assemblage in the stream test protocols for future monitoring.
Materials and Methods
The study was carried out on Chikke streams Bida Niger State. Bida is located in North Central Nigeria (9°05′N 6°01′E / 9.083°N 6.017°E). The study sites lie in the savannah region of North Central Nigeria (Fig. 1). Bida is characterized by two distinct seasons (wet and dry season). The wet season is from April to October, while the dry season is from November to March, which is entirely devoid of rain. The dry season reaches its peak in February to March (period of high sunlight and heat), while the rainy season reaches its peak between July and September (period of highest rainfall) (Olagoke and Olatunji, 2014). The Chikke stream flows from Kangi Makun village through new GRA (Government Residential Area) extension to 1990 bridge, Sima, Banyagi, to Darachita Area, where it forms a confluence with Landzun River in Bida, Niger state
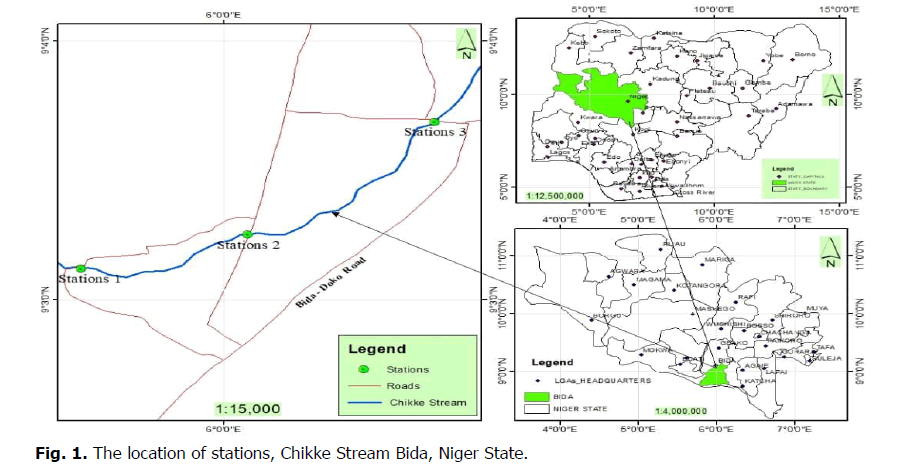
Figure 1: The location of stations, Chikke Stream Bida, Niger State.
Station 1 Chikke Stream. This is the reference point with latitude 9°3.124'N and longitude 5°59.561'E. The station is located at Kangi Makun village in new GRA extension Bida, Niger State. Few anthropogenic activities occur in this station, such as laundry, washing of plates, and farming across the stream bed. The stream bed is mostly silt and mud. Vegetation consists of several species of grasses with few Mango trees (Mangifera indica), Banana (Musa sp.), and Oil palm (Elais guineensis)
Station 2 Chikke Stream. This station is located under a motorable bridge along New Creation Academy Road Bida with a latitude of 9°3.263'N and longitude 6°0.073'E. Pumping of water for block industries is the primary activity that takes place in this station. The stream bed is sand, mud, and silt. The vegetation cover is very thick and has trees covering the surrounding. The vegetation is mixed prominent ones consist of Mango (Mangifera indica), Locust beans (Parkia biglobosa), Oil palm (Elais guineensis), and grass species
Station 3 Chikke stream. This station is located in Esozhi Area, along Federal Polytechnic road Bida with a latitude of 9°3.686'N, and longitude 6°0.601'E. It is located in an open place. Numerous anthropogenic activities occur here: laundry, car washing, washing of home utensils, pumping water by block, and heavy drainage channeled into the water body. The vegetation comprises of Mango tree (Mangifera indica), Locust beans (Parkia biglobosa), and grasses growing on the stream bank.
Samples were taken at monthly intervals over eight months (January to August 2017) between 0700 hours and 1100hours from the sampling stations. Water samples for physicochemical parameters were collected from three selected sampling stations in Chikke Stream. The sampling period covers both the dry and rainy seasons. Temperatures, depth, flow velocity, dissolved oxygen (DO), Biochemical oxygen demand(BOD5), Electrical conductivity, pH, alkalinity, turbidity, Nitrate Phosphate, and sodium were determined using standard methods and procedures (APHA, 2012).
Macroinvertebrate fauna was collected using the modified kick method (Hynes, 1961). All invertebrates collected were preserved in 40% formaldehyde and later transported to the laboratory section of the Department of Animal Biology Federal University of Technology Minna for further examination. Further analyses carried out in the laboratory include sieving (mesh size 250 µm), counting, and sorting under suitable magnifications. The macroinvertebrates were identified using manuals of Gerber and Gabriel (2002), Day et al. (2002), De Moor et al. (2003), Arimoro and James (2008), Umar et al. (2013)
The physicochemical data were analyzed by descriptive statistical test, using Microsoft Excel 2010. The mean, range, and standard deviation of each physicochemical characteristic were calculated per station. Biological indices such as taxa richness and evenness (E) abundance, number of taxa, diversity index, dominance, and physicochemical variables among all stations were compared using one-way analysis of variance (ANOVA). Canonical correspondence analysis (CCA) was used to determine the relationship between macroinvertebrate communities and environmental variables using PAST statistical software.
Results
In the Chikke stream, the depth, flow velocity, and sodium differed significantlys (p<0.05) among all the sampling stations, as indicated by ANOVA. There was no significant difference (p>0.05) in temperature, dissolved oxygen, biochemical oxygen demand, pH, turbidity conductivity, alkalinity, nitrate, phosphate, and sodium among the sampling stations of the stream (Table 1).
Table 1. Mean summary of Environmental Variables Measured at all the Sampling Stations of Chikke Stream in Bida, Niger State Nigeria (January-August, 2017).
| Environmental variables | Station 1 | Station 2 | Station 3 | Probabilities | Maximum permissible limit | ||
|---|---|---|---|---|---|---|---|
| Months | Stations | FEPA1 | SON2 | ||||
| Temperature (ºC) | 26.52±0.31 | 25.82±0.21 | 25.78±0.23 | 0.15 | 0.089 | ||
| (25.3-27.8) | (25.1-26.7) | ( 24.6-26.9) | |||||
| Flow velocity (m/s) | 0.31±0.006 | 0.31±0.009 | 0.35±0.017* | 0.448 | 0.005 | ||
| (0.29-0.34) | (0.27-0.34) | (0.28-0.41) | |||||
| Depth (cm) | 25.86±1.08 | 24.63±1.32 | 19.8±1.21* | 0.405 | 0.027 | ||
| (20.6-28.6) | (21.2 – 29.8) | (13.0-23.8) | |||||
| pH | 6.91±0.25 | 7.06±0.32 | 7.12±0.25 | 9.02E-08 | 0.867 | 6.0-9.0 | 6.5-8.5 |
| (6.0 -8.0) | (6.06 -8.90) | (6.09-8.2) | |||||
| DO (mg/l) | 5.77±0.74 | 5.92±0.66 | 6.31±0.86 | 1.15E-06 | 0.876 | 5 | |
| (2.6 -8.0) | (2.8 -8.4) | (2.4-8.8) | |||||
| BOD (mg/l) | 3.57+ 0.35 | 4.12 ± 0.36 | 4.36±0.36 | 0.003 | 0.257 | 10 | |
| (2.0 – 5.0 ) | (3.0-6.0) | (3.0-6.0) | |||||
| Turbidity (NTU) | 128.25±21.0 | 131 ± 22.2 | 135.62±24.1 | 1.11E-06 | 0.973 | ||
| (59 - 210) | (502-230) | (55-230) | |||||
| Conductivity (µ/sm) | 68.125±12.70 | 70.625±14.255 | 87.625±16.95 | 1.07E-05 | 0.603 | 1000 | |
| (20 – 122 ) | (20-129) | (20-136) | |||||
| Alkalinity (mg/l) | 16.5±3.04 | 21.5+2.66 | 21.75±2.08 | 0.003 | 0.301 | ||
| (10-30 ) | (10-30 ) | (12-30) | |||||
| Nitrate (mg/l) | 2.58±0.34 | 2.78 ± 0.43 | 2.65±0.41 | 1.59E-10 | 0.934 | 20 | 50 |
| (1.08-3.89) | (1-4.6) | (0.99-4.4) | |||||
| Phosphate (mg/l) | 0.73±0.07 | 0.70 ±0.06 | 0.67±0.07 | 3.71E-09 | 0.895 | 5 | |
| (0.45-1.86) | (0.45 – 0.87) | (0.67±0.99) | |||||
| Sodium (mg/l) | 19.1 ±0.86* | 8.45±.40 | 9.02±1.74 | 7.48E-09 | 2.20E-05 | ||
| (15.6-22.6) | (0.51-12.9) | (0.9-18.5) | |||||
Note: Values are mean±S.E; range in parenthesis values with an asterisk (*) differs significantly, 1Nigeria Water Quality Standard for Inland Surface Waters, Federal Environmental Protection Agency (FEPA, 1991), 2Nigerian Standard for Drinking Water Quality, Standard Organisation of Nigeria (SON, 2007).
A total of 719 individuals from 35 species and 24 families of macroinvertebrates were recorded during the study period in the Chikke stream (Table 2). The total number of individuals recorded In the Chikke stream per station was 251 (34.91%), 258 (35.88%), and 210 (29.20%) for stations 1, 2, and 3, respectively. The percentage distribution and abundance of taxonomic level reveal that Coleopterans, Odonata, and Hemiptera were the most common groups encountered in the stream. Ephemeroptera was sparingly found in all stations except its absence in Station 1 of the stream. Other groups were also found in good numbers (Fig. 2). In Fig. 3, the total number and percentage of individuals of macroinvertebrates recorded during the study period in Chikke stream 68% (489 individuals) were recorded in the dry season (January to April 2017), and 32% (230 individuals) were recorded in the rainy season (May to August 2017).
Table 2. Distribution and Abundance of Macroinvertebrates in Chikke Stream Bida, Niger State, Nigeria from January to August 2017.
| Order | Family | Taxa | 1 | Stations 2 |
3 |
|---|---|---|---|---|---|
| Coleoptera | Dysticidae | Phylodyte sp. | 0 | 14 | 4 |
| Notonelidae | Hydrocanthus sp. | 0 | 0 | 1 | |
| Hydrophilidae | Crenis sp. | 3 | 0 | 0 | |
| Hydrophilus sp. | 38 | 28 | 17 | ||
| Helochare sp. | 5 | 3 | 0 | ||
| Curculionidae | Unknown species | 1 | 1 | 0 | |
| Gyrinidae | Orectochilus sp. | 0 | 1 | 0 | |
| Ephemeroptera | Baetidae | Bugilesia sp. | 0 | 6 | 6 |
| Cloeon sp. | 0 | 2 | 0 | ||
| Odonata | Coegnoridae | Coenagrion sp. | 8 | 4 | 6 |
| Pseudogrian sp. | 19 | 18 | 6 | ||
| Plactinecmidae | Mesocnemis sp. | 16 | 21 | 10 | |
| Gomphidae | Gomphus sp. | 1 | 3 | 9 | |
| Ophiogomphus sp. | 34 | 28 | 20 | ||
| Aeshnidae | Aeshna sp. | 21 | 27 | 19 | |
| Cordullidae | Epitheca sp. | 3 | 3 | 7 | |
| Cordullex sp. | 0 | 0 | 5 | ||
| Libellubidae | Libellula sp. | 4 | 25 | 0 | |
| Zyxomma sp. | 2 | 3 | 6 | ||
| Brachythermis sp. | 1 | 0 | 0 | ||
| Hemiptera | Nepidae | Ranatra sp. | 2 | 3 | 1 |
| Laccocotrephes sp. | 7 | 3 | 1 | ||
| Barborophilus sp. | 4 | 4 | 3 | ||
| Naucoridae | Macrocroris sp. | 14 | 22 | 10 | |
| Naucoris sp. | 4 | 11 | 3 | ||
| Notonectidae | Notonecta sp. | 4 | 0 | 3 | |
| Gerridae | Gerris sp. | 0 | 5 | 11 | |
| Diptera | Chironomidae | Chironomus sp. | 20 | 9 | 32 |
| Culicidae | Culex sp. | 13 | 6 | 0 | |
| Ephydridae | Ephydrida sp. | 4 | 0 | 0 | |
| Mollusca | Planorbidae | Armiger crista | 0 | 3 | 1 |
| Valvatidae | Valvata tricarinata | 1 | 0 | 1 | |
| Lymnaedidae | Stanicola sp. | 0 | 5 | 11 | |
| Oligochaettes | Lumbriculidae | Lumbricoides sp. | 22 | 0 | 17 |
| Grand Total | 251 | 258 | 210 |
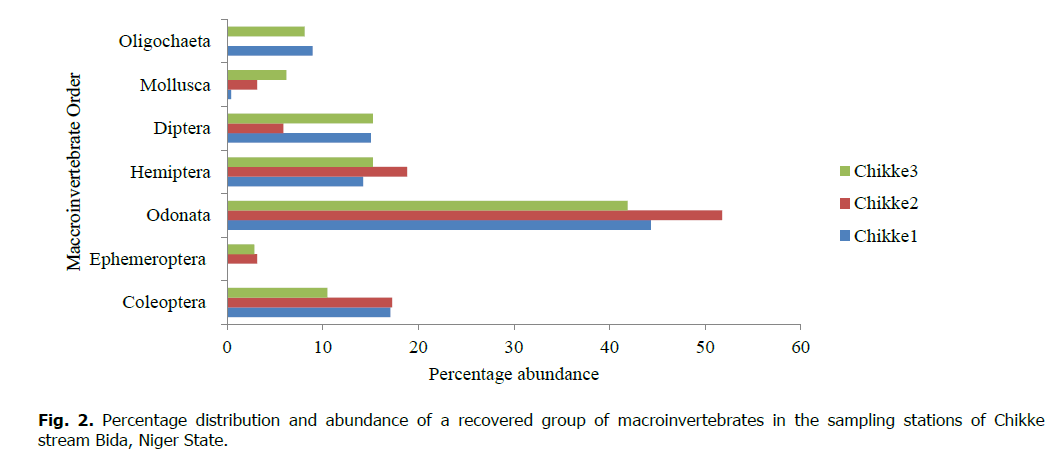
Figure 2: Percentage distribution and abundance of a recovered group of macroinvertebrates in the sampling stations of Chikke stream Bida, Niger State.
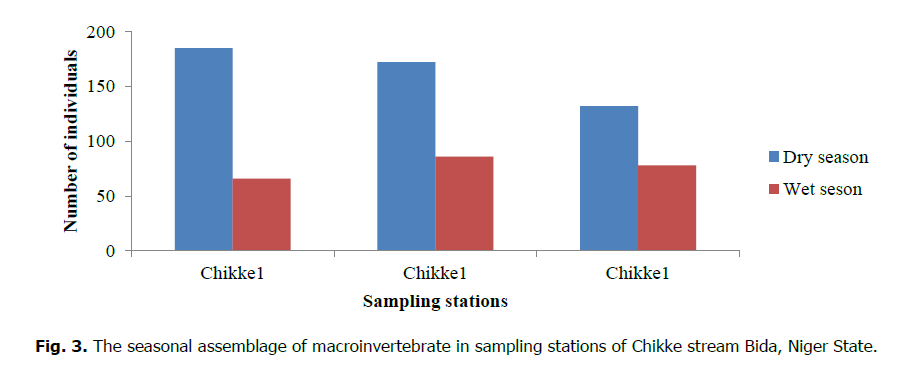
Figure 3: The seasonal assemblage of macroinvertebrate in sampling stations of Chikke stream Bida, Niger State.
Diversity, evenness, dominance, and similarity indices of Chikke stream Bida, Niger State.
Summary of biological indices, including the abundance of taxa, Shannon-Weiner diversity, evenness, and Margalef’s indices calculated for each stream station, are shown in Table 3. In the Chikke stream, the highest number of species (27) was recorded in Station 2, while 25 species were recorded in Station 1 and Station 3 respectively, Number of individuals was highest in Station 2 (258), followed by Station 1(251) and station 3(210). Simpson and Shannon's index were similar in Station 2 and Station 3, while the lowest was recorded in Station 1. Station 3 (0.7002) recorded highest Evenness index, while Station 2(0.6465) and Station 1(0.6274) recorded low indices. The evenness index shows a significant difference among sampling stations (p<0.05). Margalef’s index was recorded highest (4.682) in Station 2 followed by station 3(4.488), and the lowest (4.344) was recorded in Station 1.
Table 3. Diversity indices of the recovered benthic macroinvertebrates of Chikke stream Bida, Niger State.
| Index | Chikke 1 | Chikke 2 | Chikke 3 | |
|---|---|---|---|---|
| Taxa number | 25 | 27 | 25 | |
| Individuals number | 251 | 258 | 210 | |
| Simpson index | 0.9182 | 0.928 | 0.9285 | |
| Shannon | index | 2.753 | 2.86 | 2.862 |
| Eveness index | 0.6274 | 0.6465 | 0.7002 | |
| Margalef index | 4.344 | 4.682 | 4.488 | |
| Dominance | 0.08182 | 0.07196 | 0.07147 | |
Relationship between macroinvertebrate and measured physicochemical parameters
In the Chikke stream, the CCA tri-plot shows a positive correlation between the measured environmental variables and species abundance; the first CCA axis accounted for over 21.01% of the variation in the data set. Axis 2 accounted for 19.02% of the variation in the data set.
Organism in Axis 1 was affected mainly by Conductivity, Turbidity, Biochemical Oxygen Demand, pH, and flow velocity. CCA Axis 2 accounted for 19.58% of the variation in the data set, an organism on Axis 2 was affected by Dissolved oxygen, Total hardness, Nitrate, and Sodium, as shown in Fig. 4.
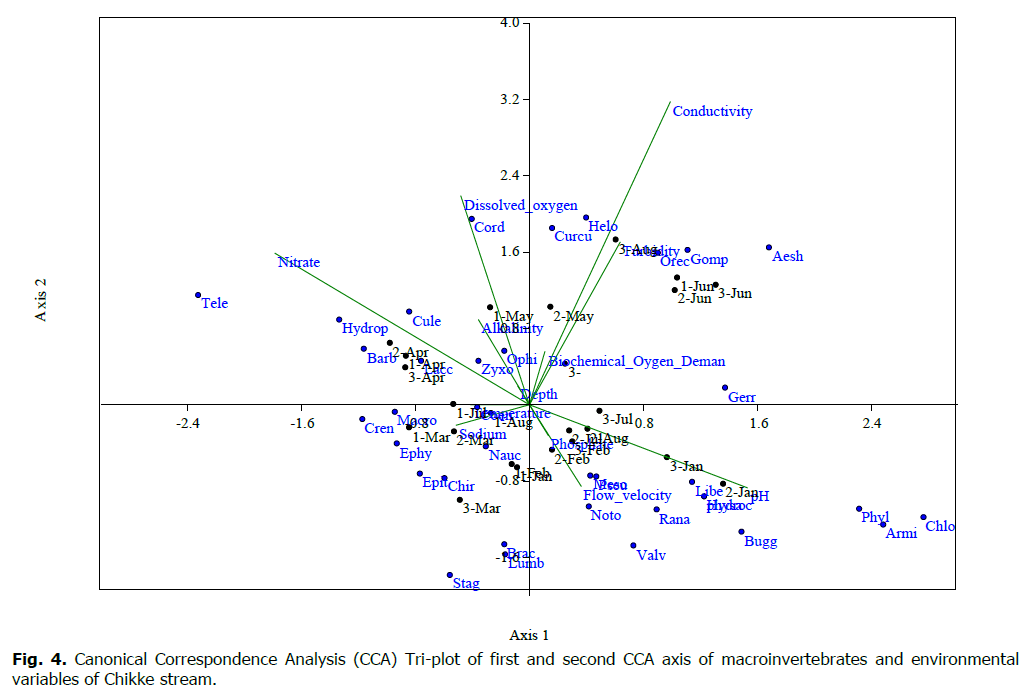
Figure 4: Canonical Correspondence Analysis (CCA) Tri-plot of first and second CCA axis of macroinvertebrates and environmental variables of Chikke stream.
Organisms associated with Axis 1 of CCA are Gomphus sp. Ophiogomphus sp., Aeshna sp. Epitheca sp. Curculionidae Cordullex sp. Helochares sp. Libellula sp. Zyxomma sp. Brachythermis sp. Ranatra sp. Macrocoris sp. Notonecta sp. Gerris sp Orectochilus sp., Armiger crista Valvata tricarinata, Stanicola sp., Lumbricoides. Organisms associated with Axis 2 of the CCA in the Chikke stream are Laccocotrephes sp., Barborophilus sp., Culex sp, and Ephydridae Philodyte Crenis sp., Hydrophylus sp., Hyphydrus sp, Bugillesia sp. Cloeon sp., Coenagorion sp., Teleganonidae Pseudogrian sp. Mesocnemis sp., Chironomus sp. and Naucoris sp (Table 4).
Table 4. Weighted intraset correlation of environmental parameters with CCA axes in Chikke Stream Bida, Niger State Nigeria.
| Environmental variables | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Eigen value | 0.47867 | 0.44609 | 0.35905 |
| % | 21.01 | 19.58 | 15.76 |
| Temperature | -0.1034 | 0.00032 | -0.4079 |
| Depth | -0.0207 | 0.04981 | -0.2628 |
| Flow_velocity | 0.09103 | -0.2141 | -0.0251 |
| Dissolved_oxygen | 0.1204 | 0.54777 | -0.3308 |
| Biochemical_Oygen_Demand | 0.02727 | 0.13982 | -0.2359 |
| pH | 0.38262 | -0.2179 | 0.03266 |
| Alkalinity | -0.0895 | 0.2234 | 0.20822 |
| Turbidity | 0.15939 | 0.4268 | 0.4187 |
| Conductivity | 0.24728 | 0.79468 | 0.4312 |
| Nitrate | -0.4466 | 0.3974 | 0.27314 |
| Phosphate | 0.03339 | -0.0812 | 0.52351 |
| Sodium | -0.1289 | -0.054 | 0.26716 |
Discussion
Physicochemical parameters
The physical and chemical properties of the stream showed some variations. However, there was no observation between the sampling stations studied. A slight variation in water level was observed. The water level of the aquatic ecosystem is usually influenced by the rainfall and pattern of the drainage basin (Ikusima et al., 1982; Emere & Nasiru, 2009). Temperature is an important environmental variable because it regulates aquatic organisms' physiological behavior and distribution (Mustapha, 2008; Anyanwu,2012). The medium temperature observed from this study could be due to the time of sample collection and the nature of vegetation around the streams. Depth ranges between 13.0 to 29.8cm, which is an indication of a shallow stream. There is a sharp increase in depth during the wet seasons as a result of rainfall. Flow Velocityrecorded in this study ranged 0.44-0.23m/s High flow regime was observed in the stream, and flow regimes increased down the stream, and significantly high values were recorded in the rain than in the dry season. This could be due to an increase in the water volume due to rain and wind blowing across the streams, which was absent during the dry season (Arimoro et al., 2015).
The pHvalue observed ranged from 6.3 to 8.9, slightly exceeding the recommended range of 6.5 to 8.5 (SON, 2007). This study's high pH values could result from surface runoff or decay of organic matter in the water (Mustapha, 2008). This finding agreed with Raji et al. (2015), who recorded a similar pH value from River Sokoto, Northwestern Nigeria. Similarly, Arimoro and Keke (2016) reported a pH range of 6.10 to 7.70 from the Gbako River.
Dissolved oxygen values ranged between 2.6 to 8.10 mg/l, which indicates a well-oxygenated water body. Higher DO values were recorded in raining season than in the dry season; this could be due to increased water volume in raining season and lower temperatures (Raji et al., 2015). This study's findings conform to Dimowo (2013), who reported DO range of 2.9-7.7mgl on his work on River Ogun southwestern Nigeria. Similarly, Keke et al. (2015) also reported a DO range of 3.5-8.2mg/l from surface water of Kaduna River Zungeru Niger state, Nigeria.
Biochemical Oxygen Demand (BOD) recorded in this study ranged between 3.0 and 7.0mg/l and falls within the maximum permissible limit (FEPA, 1991; SON, 2007). The study observed higher BOD values in the rainy season than in the dry season, resulting from the influx of organic matters into the stream through surface runoff and erosion (Mustapha, 2009; Abowei and Ekubo, 2011). This finding is consistent with the findings Arimoro and Keke (2016) reported a BOD range of 2.20-6.0mg/l from their works in Gbako River, North Central Nigeria. BOD values recorded in the study are an indication that the river was grossly polluted by organic waste (Arimoro et al., 2015).
Turbidity values ranged between 52 and 285 NTU from both streams. Higher turbidity values were recorded in the rainy season than in the dry season; this could be due to surface runoff from stream banks and sediment type (Mustapha 2008). Ayoade and Olusegun (2012) also recorded a 51.6-69.17 NTU turbidity value range from the Tropical Rivers in Southwestern Nigeria. Similarly, Ibezute et al. (2016) reported a turbidity range of 2-468NTU from Ikpoba River Edo state, Nigeria.
Conductivity ranged between 20 and 136µS/cm. A lower conductivity value was observed in the dry season than in the rainy season, indicating that the sampling station might contain more suspended and dissolved solid materials, increasing the concentration of cation such as calcium, magnesium, and sulfate (Mustapha, 2008; Anyanwu,2012). The conductivity range obtained in this study was higher than Ibezute et al. (2016), also reported a conductivity value range of 18-93µS/cm from Ikpoba River Edo State.
Alkalinity values ranged between 8.0 to 30mg/l. Higher Alkalinity value was observed in the rainy season than in the dry season; this could be due to surface runoff from nearby farms and dissolution of calcium carbonate in the water column (Yalav, 2013). Similarly, Ibemenuga et al. (2017) reported alkalinity of 7.65-21.13mg/l in the Rainforest water freshwater Ecosystem of southern Nigerian.
High Nitrate and phosphate content could result from different anthropogenic activities and surface runoff from farms and decomposition of organic matter into the water (Ibrahim et al., 2009). Phosphate is one of the limiting factors of environmental variables because, when used up, the aquatic environment can be unproductive (Arimoro et al., 2015).
Macroinvertebrate groups assemblage and distribution.
A total of 741 individuals from 34 species and 24 families of macroinvertebrates were recorded in the Chikke stream. The high abundance of individuals and diversity of macroinvertebrates in this study could be attributed to habitat structure, including vegetation, substrate type, and vegetation cover (Arimoro et al., 2015). Nutrient availability, nature of vegetation, canopy cover, and substrate type could be attributed to favoring diverse groups of macroinvertebrates (Odume et al., 2012; Arimoro et al., 2015). Surface runoff or organic materials washed into the river also favored the distribution of certain groups (Arimoro and Keke, 2016)
The Coleopterans were registered, and some of the groups were recorded in abundance. The groups encountered include Hydrophilidae, the most abundant group, Dysticidae, Notonelidae, Curculionidae, and Gyrinidae are all found across the stream areas that have more nutrient content. The Area is moderately polluted. Arimoro and Keke (2016) reported that the abundance of some coleopteran groups indicates gross pollution-free.
The Baetidae family and Teleganonidae only represented Ephemeroptera in this study. Two species of the Baetidae family were encountered, Bugillesia sp. and Cloeon sp. The low abundance of Ephemeroptera in this study results from their sensitivity to a polluted environment and the deteriorated state of the stream due to different anthropogenic activities (Arimoro and Ikomi, 2008). Using the EPT index, Plecoptera and Trichoptera were absent throughout the study period. The low abundance of Ephemeroptera and absence of Plecoptera and Trichoptera in the sampling stations is an indication of gross pollution due to anthropogenic activities at the stations, since many studies have reported higher abundance and diversity of this group of macroinvertebrate to clean and pollution-free water bodies (Arimoro and Ikomi, 2008; Odume et al., 2012).
The abundance of Odonata in the stream is an indication of poor water quality. Odonatan’s are moderately tolerant to pollution. Most families in this group were found in all the stream sampling stations either as a result of the vegetation cover and bottom sediment of the streams favoring their colonization (Arimoro et al., 2015). The encountered families were Coegnoridae comprising of Coenagrion sp. and Pseudogrian sp., Platycnemididae comprising of Mesocnemis sp, Aeshnidae comprising of Aeshna sp, Cordullidae comprising of Epitheca sp. and Cordullex sp and Libellubidae which consist of Libellula sp. Zyxomma sp. and Brachythermis leucostica and the family Gomphidae was consisting of Gomphus sp and Ophiogomphus sp. Similar findings have also been reported in Nigeria by (Arimoro and Ikomi 2008; Emere and Nasiru 2009; Edegbene et al., 2015).
Hemiptera was also found in the Chikke stream; their diversity and abundance on the stream due to the vegetation cover and the bottom sediment of the streams favoring their colonization, and was represented by families such as Nepidae; Ranatra sp, Lacocotrephes sp, and Boborophilus sp. Naucoridae; Macroris sp, Naucoris sp, Notonectidae represented by Notonecta sp only and gerridae represented by Gerris sp only. Similar studies reported the presence of hemipterans from their works on Nigeria Freshwater (Arimoro and Ikomi, 2008; Emere and Nasiru, 2009; Arimoro et al., 2015; Arimorso and Keke, 2016). Favorable environment variables such as substrate type, vegetation covers are the factors responsible for increased species richness and diversity of subtropical African waters (Arimoro et al., 2015).
In this study, the presence of Dipterans indicates gross pollution caused by decaying organic waste in the water body, as reported by (Edegbene et al., 2015). Dipterans were represented by the families Chironomidae (midges), which include Chironomus sp. Family Culicidae, Culex sp and Ephydridae. Mollusca and Oligochaeta are primarily found in polluted areas of water bodies because they are very tolerant of pollution. Their presence is supported by favorable environment variables such as substrate type and vegetation covers. Mollusca were represented by the family Planobidae; Armiger crista, Valvatidae, Valvatia tricannata, Lymnaedidae. The family Lumbricoides represented Oligochaeta. Their presence has been reported in some freshwater studies in Nigeria (Emere and Nasiru, 2009; Dadi-Mamud et al., 2014)
There is a positive correlation between the measured environmental variable and macroinvertebrates species presence in Chikke streams. The scarcity of Ephemeroptera, the absence of Trichoptera and Plecoptera, indicates pollution, which also signifies the deterioration of the biotic and overall ecological health of the river. Several researchers have reported the absence or low abundance of this group in Nigerian water bodies (Arimoro et al., 2015; Edegbene et al., 2015; Arimoro and Keke, 2016). Species richness, diversity, and evenness indices of each sampling station of the streams during the sampling period reflect the water quality condition of each stream. Seasonally there was variation in macroinvertebrate abundances and composition during the sampling periods. High macroinvertebrate abundance was encountered during the dry season than in the wet season. This could be due to increased water volume during the wet season, increased flow characteristics, and surface runoff from the surrounding environment, which must have erupted the habitat structure in the wet season. Similarly higher abundance of macroinvertebrates was recorded in the dry season in many streams of Nigeria (Arimoro and Ikomi, 2008; Keke et al., 2017)
References
Abowei, J. E. N. & Ekubo, A. T. (2011). Aspect of Aquatic pollution in Nigeria. Research Journal of environmental and earth sciences, 3 (6), 676-693.
Amini-Yekta, F., Kiabi, B., Ardalan, A. & Shokri, M. (2013). Temporal Variation in Rocky Intertidal Gastropods of the Qeshm Island in the Persian Gulf. Journal of Persian, Gulf, 4 (13), 9-18.
Andem, A. B., Okorafor K. A., Eyo V. O. & Ekpo, P. B. (2014). Ecological impact assessment and limnological characterization in the intertidal region of Calabar River using benthic Macroinvertebrates as bioindicator organisms. International Journal of Fish Aquaculture Studies, 1 (2), 8–14.
Anyanwu, E. D. (2012). Physico-Chemical and Some Trace Metal Analysis of Ogba River, Benin City, Nigeria. Jordan Journal of Biological Sciences, 5 (1), 47-54.
APHA (American Public Health Association) (2012). Standard methods for examination of water and wastewater. Maryland U.S.A. United Book Press Inc. Baltimore.
Arimoro, F. O. & Ikomi, R. B. (2008). Response of macroinvertebrates to abattoir wastes and other anthropogenic activities in a municipal stream in the Niger Delta, Nigeria. Environmentalist, 28, 85-98.
Arimoro, F. O. & James, H. M. (2008). Preliminary pictorial guide to the macroinvertebrates of Delta State Rivers, southern Nigeria. Albany Museum, Grahamstown.
Arimoro, F. O. & Keke, U. N. (2016). The intensity of human-induced impact on the distribution and diversity of Macroinvertebrates and water quality of Gbako River, North Central Nigeria. Energy Ecology and Environment, 16 (8), 25-36.
Arimoro, F. O., Chukwujindu, M. A., Iwegbue B. & Enemudo, O. (2012). Effects of Cassava effluent on benthic macroinvertebrate assemblages in a Tropical Stream in Southern Nigeria. Acta Zoologica Lituanica, 18 (2).
Arimoro, F. O., Ikomi, R. B., Ajuziego, I. O. & Nwadukwe, F. O. (2011). Temporal and spatial variability in the structure of macroinvertebrates communities and environmental variables of a Niger Delta Creek. African Journal of Aquatic Sciences, 36, 57-66.
Arimoro, F. O., Odume, N. O., Uhunoma, S. I. & Edegbene, A. O. (2015). Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Environmental Monitoring Assessment. 187, 1–14.
Ayoade, A. A. & Olusegun, A. O. (2012). Impacts of Effluents on the Limnology of a Tropical River, Southwestern Nigeria. Journal of Applied Science and Environment Management, 16 (2), 201–207.
Dadi-Mamud, N. J., Oniye, S. J., Auta, J. & Ajibola, V. O. (2014), Community pattern and diversity index Of Macro-Invertebrates in relation to surface water interface of River Ndakotsu, Lapai Nigeria. Asian Journal of Science and Technology. 5 (9), 546-552.
Day, J. A, Harrison A. D., & de Moor I. J., (2002). Guides to the freshwater invertebrates of Southern Africa, volume 9. Diptera TT 201/02 Pretoria: Water Research Commission.
De Moor, I. J., Day, J. A., & De Moor, F. C. (2003). Guides to the freshwater invertebrates of Southern Africa, volume 7. Insecta I: Ephemeroptera, Odonata and Plecoptera. Prepared by Water Research Commission, Pretoria, South Africa.
Dimowo, B. O. (2013). Assessment of Some Physico-chemical Parameters of River Ogun (Abeokuta, Ogun State, Southwestern Nigeria) in Comparison With National and International Standards. International Journal of Aquaculture, 3 (15), 79-84.
Edegbene, A. O., Arimoro, F. O., Odoh, O. & Ogidiaka, E. (2015). Effect of anthropogenicity on the composition and diversity of aquatic insect of a municipal River North Central Nigeria. Bioscience Research in Today’s World, 1 (1), 55-66.
Edokpayi, A. C., Olowoporoku, A. O. & Uwadiae, R. E. (2010). The hydrochemistry and macrobenthic fauna characteristics of an urban draining creek. International Journal of Biodiversity and Conservation 2(8), 196-203,
Emere, M. C., & Nasiru, C. E. (2009). Macroinvertebrate as Indicator of the water quality of an Urbanized stream, Kaduna Nigeria. Journal of aquatic sciences, 7, 2-3.
Federal Environmental Protection Agency (FEPA, 1991) Guideline and standards for Environmental pollution control in Nigeria. Federal Environmental Protection Agency, 27, 20
Garcia-Roger, E. M., Sánchez-Montoya, M. M., Gomez R, Suárez, M. L., Vidal-Abarca, M. R., Latron, J., Rieradevall, M. & Prat, N. (2011) Seasonal changes in habitat features influence aquatic macroinvertebrate assemblages in perennial versus temporary Mediterranean streams. Aquatic Sciences, 73, 567–579.
García-Roger, E. M., Sánchez-Montoya, M. M., Gomez R, Suárez, M. L., Vidal-Abarca, M. R., Latron, J., Rieradevall, M. & Prat, N. (2011) Seasonal changes in habitat features influence aquatic macroinvertebrate assemblages in perennial versus temporary Mediterranean streams. Aquatic Sciences, 73, 567–579.
Gerber, A. & Gabriel, M. J. M. (2002). Aquatic invertebrates of South African Rivers field guide. Institute of water quality studies South Africa.
Giam, X. & Olden, J. D. (2016). Environment and predation governing fish community assembly in temperate streams. Global Ecology & Biogeography. 5.
Hynes H. B. M. (1961). The Invertebrate fauna at Welsh Mountain Stream. Archive Hydrobiology., 57, 344-388.
Ibemenuga, K. N. & Nzekwe, O. I. (2017). Effects of Cassava (Manihot esculenta) Effluents on Macroinvertebrate Assemblages in a Rainforest Lotic Freshwater Ecosystem, Southern Nigeria. Tropical Journal of Applied Natural Sciences, 2 (1), 46-53.
Ibezute, A. C., Asibor, G. I. & Ibezute, G. I. (2016). Ecological Assessment of Brewery Effluent Impact on the Macrobenthic Invertebrates of Ikpoba River, Edo State, Nigeria International Journal of Ecosystem, 6 (3), 47-54
Ibrahim, B. U., Auta J. & Balogun J. K. (2009). Assessment of the physicochemical parameters of Kontagora Reservoir Niger state. Bayero Journal of pure and applied sciences, 2 (1), 64-69.
Ikusima, I., Lim, R.P. & Furtado J. I. (1982). Environmental conditions in: J.I. Futado and S Mori(eds). Tasek Bera the Ecology of a Tropical Freshwater swamp. Dr. W. Junk, The Hague. Pp 55-148.
Keke, U. N., Arimoro, F. O., Auta, Y. I. & Ayanwale, A. V. (2017). Temporal and spatial variability in macroinvertebrate community structure in relation to environmental variables in Gbako River, Niger State, Nigeria. Tropical Ecology, 58 (2), 229–240.
Keke, U. N., Arimoro, F. O., Ayanwale, A. V. & Aliyu, S. M. (2015). Physicochemical parameters and Heavy Metals content of surface water in downstream Kaduna River, Zungeru, Niger state, Nigeria. Applied science research Journal, 3 (2), 46-57.
Kun, Li, Chunguang, H., Jie, Z., Zhenxing, Z., Hongyong, X., Zhongqiang, W., Haijun, Y. & Lianxi, S., (2015). Long-term changes in water quality and Macroinvertebrates communities of a subtropical river in south China. Water 2015, 7, 63-80.
Linares, M. S., Faccioli, G. G. & Freitas, L.M. (2013) Benthic macroinvertebrate community structure and seasonal variation in a neotropical stream in the State of Alagoas, Brazil. Biota Neotropical, 13 (3).
Marques, M.J., Martinez –Conde, E. & Rovira, J.V. (2003). Effects of zinc and lead mining on the benthic macroinvertebrate fauna of a fluvial Ecosystem. Water Air and soil pollution. 148:363-388.
Mustapha, M. K. (2008). Assessment of the Water Quality of Oyun Reservoir, Offa, Nigeria, Using Selected Physico-Chemical Parameters Turkish Journal of Fisheries and Aquatic Sciences 8, 309-319.
Nicola, G. G., Almodóvar, A. & Elvira, B. (2010). Effects of environmental factors and predation on benthic communities in headwater streams. Aquatic Science, 72, 419-429.
Nkwoji J. A., Yakub, A., Ajani, G. E., Balogun, K. J., Renner, K. O., Igbo, J. K., Ariyo, A. A. & Bello, B. O. (2010). Seasonal variations in the water chemistry and benthic macroinvertebrates of a south western Lagoon, Lagos, Nigeria. Journal of American Science, 6(3), 85-92.
Nyenje, P., M., Foppen, J. W., Uhlenbrook, S., Kulabako, R. & Muwanga, A. (2010). Eutrophication and nutrient release in urban areas of sub-Saharan Africa- A review. Science & Total Environment, 408, 447–455.
Odume, O. N., Muller, W. J., Arimoro, F. O., & Palmer, C. G., (2012). The impact of water quality deterioration on macroinvertebrate communities in the Swartkops River, South Africa: a multimetric approach, African Journal of Aquatic Science, 37 (2), 191-200.
Raji, M. I. O., Ibrahim, Y. K. E., Tytler, B. A. & Ehinmidu, J.O. (2015). Physicochemical Characteristics of Water Samples Collected from River Sokoto, Northwestern Nigeria. Atmospheric and Climate Sciences, 5, 194-199.
SON, (2005). Nigerian Standard for Drinking Water, Nigerian industrial standard NS554, Standard organisation of Nigeria Lagos. 30pp.
Umar, D. M., Hardling J. S. & Winterbourn, M. J. (2013). Photographic guide of freshwater Invertetbrates of the Mambilla Plateau Nigeria. Published by School of Biological Sciences University of Canterbury, New Zealand.
WHO (2008). Guidelines for drinking-water quality: incorporating 1st and 2nd addenda, Vol.1, recommendations, 3rd ed. World Health Organization. Available from: https://apps.who.int/iris/handle/10665/204411/
Wibowo, D. N. & Santoso, S. (2017). Benthic macroinvertebrate diversity as biomonitoring of organic pollutions of river ecosystems in Central Java, Indonesia. Biodiversitas, 18, 671-676.
Zajac, R. N., Vozarik, J. M. & Gibbons, B. R. (2013). Spatial and temporal patterns in macrofaunal diversity components relative to sea floor landscape structure. PLoS ONE, 8 (6).
Author Info
Y.M. Mohammed1*, F.O. Arimoro1, A.V. Ayanwale1, K.M. Adamu2, U.N. Keke1, M.D. Abubakar1, A.C. Achebe<1 and A.C. Achebe<12Biology Department, Ibrahim Badamasi Babangida University Lapai, Niger state, Nigeria
Citation: Mohammed, Y.M., Arimoro, F.O., Ayanwale, A.V., Adamu, K.M., Keke, U.N., Abubakar, M.D., Achebe, A.C. (2021). The current state of water quality and benthic invertebrate fauna in Chikke Stream (North-Central Nigeria). Ukrainian Journal of Ecology, 11 (3), 26-34.
Received: 12-Apr-2021 Accepted: 26-May-2021 Published: 31-May-2021, DOI: 10.15421/2021_138
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
