Research - (2021) Volume 0, Issue 0
Specific adaptation features of Hedysarum gmelinii Ledeb. (Fabaceae Lindl.) in the mountains of South Siberia
E.V. Zhmud1*, N.B. Williams2, Y.S. Bukin3 and O.V. Dorogina1Abstract
Pathogenesis-related protein inhibitors of trypsin (PR-6 proteins) are one of 16 factors of immunity in angiosperms. Their activity in the aboveground parts of plants ensures preservation of nonspecific systemic resistance to unfavorable biotic or abiotic environmental factors. Trypsin inhibitor activity (TIA) has hardly been studied in leaves of wild representatives of Fabaceae Lindl. For the first time, studies of background values of TIA in the leaves of intact mature generative individuals of Hedysarum gmelinii Ledeb. in the mountains of Altai and Krasnoyarsk Territories, the Republics of Altai and Khakassia, in the range of absolute elevations of 300-2200 m were carried out. In most of the cenopopulations, high TIA values were detected (more than 25 mg/g of dry weight of ground leaves). Higher TIA were found in representatives of the species inhabiting more extreme northern part of the studied area and in the arid highlands of Southeast Altai. TIA in H. gmelinii leaves can increase during the years of unusual weather patterns (with sharp fluctuations in the environmental temperatures).
Keywords
Hedysarum gmelinii Ledeb., Trypsin inhibitor activity (TIA), Adaptation, Variability of quantitative traits.
Introduction
It is known that environmental conditions-temperature, intensity of solar radiation, moisture, etc., have an effect on the main parameters of plant life (Khandaker et al. 2009, Sarker and Oba 2018 a, Sarker and Oba 2018 b, Re et al. 2018). The published data on adaptive biochemical changes in plants in natural conditions are relatively scarce. An important role in plant adaptation to environmental conditions and the maintenance of systemic nonspecific resistance is played by PR-6 proteins (pathogenesis-related proteins), or trypsin inhibitors (TI). These substances are synthesized in almost all angiosperms (Trifonova et al. 2007, Mosolov and Valueva 2008). The synthesis of this group of substances, together with the action of other factors of plant immunity, makes it possible to adapt to unfavorable conditions. Trypsin inhibitor activity (TIA) makes the main contribution to the formation of systemic acquired nonspecific resistance, and does not depend on the nature of the stressor (Domash et al. 2008, Kamble and Jadhav 2016, Madamanchi and Kus 1991, Kidrič and Sabotič 2014). Protein peptidase inhibitors are essential molecules that modulate peptidase activity.
Plant protease inhibitors have been studied as therapeutic agents. In the treatment of human diseases in which increased proteolytic activity is observed, protease inhibitors (PI) have high pharmaceutical potential (Gitlin-Domagalska et al. 2020). Plant-derived PIs also have been described as antineoplastic agents in immunopharmacology, as well as substances targeting proteases involved in neurodegenerative diseases (Hellinger and Gruber 2019).
It is noted in the literature that their global presence in different plant species is unknown (Chye et al. 2006). Studies of the background values of TIA in the leaves of plants are few and only had been carried out in some species of cultivated plants, mainly experimentally. They showed that in the leaves of tomatoes (Shamei et al. 1996) and Amaranthus hypochondriacus L. (Amaranthaceae) (López et al. 2004), there is an increase in TIA in response to an increase in illumination intensity. The authors of these works suggested that IT can play a protective role against temperature stress.
There are no data on TIA variability in leaves of plants in natural populations. Meanwhile, studies of TIA in various ecological and geographical conditions would expand the understanding of the adaptive potential of plant species for the prospects of their use. Species inhabiting a wide ecological and altitude range is a convenient model for such research. In the mountains, there is a significant change in the environment over relatively short distances due to redistribution of climatic factors (Mirkin and Naumova 2011, Larcher et al. 2010). With increasing altitude, numerous environmental parameters change significantly. Therefore, for the adaptation of plants in the mountains, specialization at the structural and functional levels is necessary (Kumar and Kumar 2017). The mountains of Southern Siberia are a convenient model for studying the processes of plant adaptation due to the mosaic nature of vegetation and climatic conditions concentrated on a relatively small area. These reasons determine the diversity of plant adaptive reactions in different parts of the range (Pjak 2003). The heterogeneity of climatic conditions in Altai Mountains manifests itself in increased climate aridization and an increase in absolute altitude in the direction from northwest to southeast. The Central and Northern Altai belong to transition zone from relatively favorable (warm and humid) habitats of the Northwestern Altai to the arid conditions of the highlands of Southeastern Altai (Makunina 2016).
Hedysarum gmelinii Ledeb. is a polymorphic forest-steppe species with a wide altitude range. According to the literature, this is a young species; it is in the process of further speciation based on ecological differentiation (Syeva et al. 2008). In the process of adaptation to the mountain conditions, we noted morphological plasticity in H. gmelinii. In H. gmelinii, the length of the generative shoot significantly decreases with an increase in the absolute elevation in the mountains (Zhmud 2014). Also in the leaves of H. gmelinii a wide range of TIA was identified in our previous studies (Dorogina and Zhmud 2010). We hypothesized that TIA can participate in adaptation of H. gmelinii to mountain conditions. The aim of this study is to detect the possible adaptive function of trypsin inhibitor activity in the leaves of H. gmelinii in various ecological and geographical conditions of the mountains of Southern Siberia.
Materials and Methods
During a period of 2000-2014, H. gmelinii was investigated in 25 habitats, covering the range of absolute elevations of 300-2200 m above sea level (m ab. s. l.) (Table 1 and Fig. 1). The northern low-mountainous part of the area: south of the Krasnoyarsk Territory (KT) and Republic of Khakassia (RK),-is characterized by a cool sharply continental climate (Polikarpov 1986). The central part of the species’ range is located in the Altai mountains (Altai Republic, (AR)) (Ilyina et al.2020). Here, the main studies of TIA variability have been carried out. In this part of the range, a more humid and warmer climate is observed, which changes towards higher aridization in the direction from the North-West to the South-East Altai. In the northwestern part of AR, a moderately warm, humid climate remains. In the central part of AR the climate has transitional properties, and in the high mountainous regions of the southeastern Altai, it is again characterized as arid, sharply continental (Makunina 2016). Habitat coordinates are plotted using the SAS.Planet.Release.191221 program.

Fig 1. Locations of the studied cenopopulations of Hedysarum gmelinii in the South Siberia.
| S. No* | Year of investigation; name of location; GPS coordinates | Altitude (m), slope (sl.) of exposure (exp.) | Name of phytocoenosis |
|---|---|---|---|
| North of the central part of the species’ range Krasnoyarsk Territory, Sharypovsk district | |||
| 1 | 2011; the vicinity of the village (v.o.v.) Sorokino; 55°30.917ʹ; 88°51.983ʹ | 460 m; shore of the lake Ingol, sl. of the northern exposition | Meadow |
| The Republic of Khakassia, Shirinsk district | |||
| 2 | 2011; s.l. Itkul; 54°28.878ʹ; 90°06.913ʹ | 500 m; north slope | Meadow with steppe grass species |
| 3 | 2011; v.o.v. Son; 54°22.246ʹ; 90°22.329ʹ | 500 m | Meadow |
| Altaisk Territory, Krasnoshchekinsk district | |||
| 4 | 2010; v.o.v. Chineta; 51°18.589ʹ; 83°01.122ʹ | 600 m; sl. south exp. | Meadow with steppe grass species |
| Altai Republic: North-Eastern area Chemal district | |||
| 5-a | 2010 (5_10); | 600 m; bank of the river; | Edge of a sparse Pine |
| 5-b | 2012 (5_12); v.o.v. Chemal; 51°11.459ʹ; 86°05.398ʹ | sl. south exp. | forest |
| Altai Republic: Western area Ust-Kan district | |||
| 6 | 2012; v.o.v. Black Anui; 51°23.313ʹ; 84°41.299ʹ | 700 m; sl. south exp. | Meadow |
| 7 | 2012 г.; v.o.v. White, Anui; 51°14.397 ʹ; 84°54.560 ʹ | 1000 m; sl. south-eastern exp. | Rocky steppe |
| 8 | 2003; v.o.v. Ust-Kan; 50°17.553ʹ; 85°28.651ʹ | 1100 m; terrace r. Charysh | Meadow steppe with Hedysarum and Stipa |
| 9 | 2012; v.o.v. Yakonur; 50°59.211ʹ; 84°52.275ʹ | 1100 m | degraded steppe under grazing |
| 10 | 2010; v.o.v. Abay; 50°26ʹ; 85°3333ʹ | 1300 m; south slope | Meadow |
| 11 | 2003; v.o.v. Oro; 50°54.717ʹ; 85°0033ʹ | 1300 m | Meadow steppe |
| Altai Republic: Central area Ongudai district | |||
| 12 | 2009; v.o.v. Ongudai; 50° 43.437ʹ; 86°13.437ʹ | 800 m | Meadow with steppe grass species; |
| 13 | 2000; v.o.v. Inya; 50°27.546ʹ; 86°40.253ʹ | 900 m; Chuya river (r.) terrace; sl. south exp. | Shaded steppe on gravel soil |
| 14 | 2003; v.o.v. Khabarovka; 50°41.15ʹ; 86°17.317ʹ | 1000 m; hollow between hills | Meadow steppe |
| 15 | 2000; v.o.v. Small Yaloman; 50°29.867ʹ; 86°34.567ʹ | 1100 m; Saldiar r. terrace; sl. Southwest | Grass meadow with shrubs on gravelly soil |
| 16 a | 2003 (16_03); | 1100 m | Steppe with Stipa |
| 16 b | 2012 (16_12); v.o.v. Yelo; 50°46.05ʹ; 85°33.5ʹ | ||
| 17 a | 2009 (17_09); | 1100 m; northwestern sl. | Meadow under the |
| 17 b | 2011 (17_11); | canopy of a sparse larch | |
| 17 c | 2012; (17_12); Chike-Taman Pass; 50°38.870ʹ; 85°.39.453ʹ | ||
| 18 | 2012; river Aigulak, place of confluence; 50°21.714ʹ; 87°14.764ʹ | 1100 m; south slope on the river terrace | Meadow at the edge of a sparse larch |
| 19 | 2012; v.o.v. Kulada; 50°40.460ʹ; 85°47.354ʹ | 1100 m; south slope | Degraded rocky steppe under grazing |
| 20 | 2000; Shawla river valley | 900 m; on the banks and rocky bottom of the dry riverbed | Birch-poplar forest with undergrowth |
| 21 | 2000; Shawla river valley | 1100 m; terrace r. Shawla; sl. Southwest | Steppe with shrubs on gravel soil |
| 22 | 2000; Shawla river valley | 1100 m; terrace r. Shawla | Steppe with Achnatherum splendens |
| Altai Republic: South-Eastern area Ulagan district | |||
| 23 a | 2007 (23_07); | 2000 m; southeast sl. | Alpine meadow on a rocky substrate |
| 23 b | 2010 (23_10); v.o.v. Aktash; 50°17.983ʹ; 87°43.983ʹ | ||
| Kosh-Agach district | |||
| 24 | 2009; v.o.v. Kyzyl-Tash; 50°12.167ʹ; 87°51.267ʹ | 1700 m; terrace r. Ak-Turu, south slope | Meadow at the edge of a sparse larch |
| 25 | 2003; v.o.v. Kosh Agach; 49°59.55ʹ; 88°40.55ʹ | 2200 m; Tarkaty r. terrace, east sl. | Degraded steppe |
Table 1. Characteristics of the habitats of Hedysarum gmelinii in the central part of the species’ range.
TIA was studied in 10-20 mature generative individuals without external damage, which were in the “flowering-beginning of fruiting” phase, sampled in each cenopopulation (CP) (n=372). The CP designation in the diagrams includes the ordinal number of the habitat. In four habitats in the same CP, H. gmelinii was studied for 2-3 years. These cases were considered as separate CP; and the last two digits of the year of research were added to the habitat number (Table 1). Therefore, 30 CP out of 25 habitats were studied.
Hoffman and Weisblai (1975) method was modified to determine TIA values in dried leaves (Methods of… 1987). Earlier, we found that TIA in dried leaves in some species of the genus Hedysarum is completely preserved (Zhmud et al.2018). TIA values in milligrams of pure bovine trypsin inhibitor bound per gram of air-dry leaf meal (moisture content 6%) (mg/g dry weight, hereinafter mg/g) are expressed. In our study of TIA, BAPA (Nα-benzoyl-DL-arginine-p-nitroanilide) as a substrate and bovine trypsin (manufactured by ISN-Biomedical, USA) were used. The method is based on the spectrophotometric measurement of the optical density of the decay products of BAPA under the action of trypsin, at a wavelength of 405 nm. Addition of the plant extract with trypsin inhibitors to the substrate binds some trypsin, which leads to decrease in extinction. The following buffer was used: 0.05 M Tris-HCl-0.02 M CaCl2 (pH=7.7). TIA values were considered low if measured at less than 25 mg/g, and considered high at more than 25 mg/g.
The following options for studying the dependence of TIA on abiotic environmental factors are selected:
TIA~No_location, TIA~years, TIA~Height_above_sea_level, TIA~No_location+years, TIA~No_location+Height_above_sea_level, TIA~years+Height_above_sea_level. PERMANOVA analysis (Anderson 2001) in the “R” free-domain statistical software, the "vegan" package, was used (Oksanen et al. 2010). Analysis for all possible linear combinations of TIA with abiotic environmental factors using a set of scripts was carried out (Dyson 2018). In each linear combination for the R programming language, the values of the AIC and AICc criteria were calculated according to the algorithm proposed by Dyson (2019). They determined the informational significance of a linear combination of abiotic factors. The linear combination of abiotic factors with the lowest AIC and AICc values made the largest statistically significant contribution to the determination of the TIA value.
Statistical analysis was carried out using StatSoft EXCEL; and graphic display of the results was constructed by STATISTICA software package. The variation was discussed according to the classification of Zaitsev (1984). A preliminary analysis by the Shapio-Wilk test showed that TIA values were not normally distributed in individuals from 9 out of 30 samples (P>0.05). Therefore, nonparametric Mann-Whitney test was used (1947) to determine the reliability of differences between the mean TIA values. Cluster analysis was used to identify the degree of similarity in the distribution of TIA values in plants from different regions for grouping samples with different TIA values. Clusters were made with complete linkage city-blocks (Manhattan) distances. The mean values with a 95% confidence interval were calculated in the groupings of plants identified in the cluster analysis according to the TIA values. In the plots, the variability of the means with a 95% confidence interval was shown. The statistically significant difference in means was based on P-values less than 0.05. For each CP, the arithmetic mean value of TIA (M), standard error of mean (m), maximum (max) and minimum (min) values, ratio (max/min) and coefficient of variation (Cv, %) were determined.
Results
H. gmelinii is a tap-root caudex-forming monocentric polycarpic with semi-rosette monocarpic annual shoots. Our previous studies found very high levels of inter-individual variability of the extreme TIA values (0.74-64.2 mg/g) in the leaves of different representatives of H. gmelinii. (Zhmud and Dorogina 2010). We performed cluster analysis of TIA in samples from 30 CPs in 25 habitats of H. gmelinii.
Results of the PERMANOVA analysis of the influence of abiotic factors on TIA are presented in Table 2. According to the AIC and AICc values, the linear combination of the “No_location” factor had the greatest weight. It accounted for 56.9% of the variability in TIA values. The value of the covariance coefficient in this case (R2) was significantly different from 0 (P=0.001<0.05).
| Model | R2-covariance Coefficient Value |
AIC Value | AICc Value |
|---|---|---|---|
| TIA ~ No_location | 0.569 | 3679.168 | 3684.687 |
| TIA ~ years | 0.164 | 3876.946 | 3877.257 |
| TIA ~ Height_above_sea_level | 0.001 | 3932.913 | 3932.946 |
| TIA ~ No_location+years | 0.572 | 3680.743 | 3686.475 |
| TIA ~ No_location+Height_above_sea_level | 0.570 | 3680.865 | 3686.813 |
| TIA ~ years+Height_above_sea_level | 0.165 | 3878.893 | 3879.294 |
| TIA ~ No_location+years+Height_above_sea_level | 0.570 | 3680.965 | 3686.843 |
Table 2. The results of PERMANOVA analysis to assess the effect of a complex of abiotic factors on TIA in Hedysarum gmelinii leaves. No_location-sampling area; years-sampling year; Height_above_sea_level-terrain height above sea level.
Therefore, based on the PERMANOVA analysis, it can be concluded that only this abiotic factor (the influence of conditions in the habitat) significantly influenced the TIA values in H. gmelinii leaves in our study (Table 2).
Individuals of the species were distributed in accordance with this trait into two clusters close in volume (Fig. 2a). Cluster 1 (n=200) included a group of individuals of 17 samples with high mean TIA values (30.5 ± 0.6 mg/g) (Fig. 2b). They were growing at altitudes of 300-2200 m above sea level in the northern part of the range (KT, RK), and in the Central and South-Eastern areas of AR (Table 1).
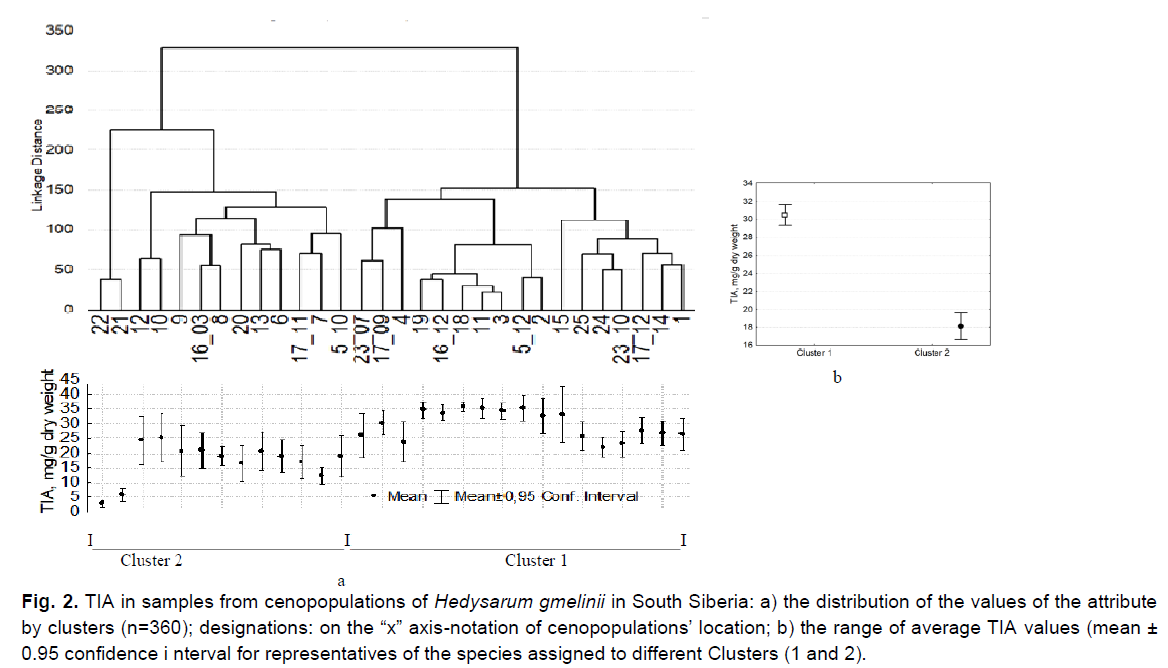
Fig 2. TIA in samples from cenopopulations of Hedysarum gmelinii in South Siberia: a) the distribution of the values of the attribute by clusters (n=360); designations: on the “x” axis-notation of cenopopulations’ location; b) the range of average TIA values (mean ± 0.95 confidence i nterval for representatives of the species assigned to different Clusters (1 and 2).
The ratio of the maximum over minimum for the CP TIA (max/min) for the samples of this
Cluster, on average, was 2.3 ± 0.2. The average TIA values over the aggregate of samples from this cluster varied within normal limits (26.9%), while in individual CPs this variability ranged from very low to very high (Table 2). Most individuals in this cluster were characterized by high average TIA values for CP (more than 25 mg/g). The minimum values in most of the samples from this cluster exceeded 10 mg/g (Fig. 2a).Individuals in 8 habitats out of 15 total habitats of Cluster 1 grew at the extreme studied points as following: in the northern part of the range-individuals from CPs 1, 2, 3; in the edge western point of the central part of the range in AR-CP No 4. In AR, habitats No 18, 23, 24, 25 are the most extreme in South-Eastern area, where H. gmelinii grew at absolute elevations above 1700 m (Table 1 and Fig. 1).
In addition to individuals from extreme geographic points and from high mountains, individuals from 7 habitats of a more favorable bioclimatic central area of AR (No 5, 11, 14, 15, 16, 17, 19) were also assigned to Cluster 1 (Makunina 2012) (Table 1). This can be explained by the unequal TIA levels in the leaves of plants studied in different years with different weather conditions. Individuals from four AR habitats (No 5, 16, 17, 19) have been studied by us in different years. Subsequently, individuals from three CP (No 5, 16, 17) were assigned to different clusters (Fig. 2a). This is due to the presence of annual TIA variability in individuals of the same habitat. Studies have shown that the TIA values in representatives in each of these three locations in different years differed 1.5-1.8 times (P=0.000-0.009<0.05; Table 3).
| Number of Location |
Number of Location |
p-value |
|---|---|---|
| 5a | 5b | 0.0001 |
| 16a | 16b | 0.001 |
| 17a | 17b | 0.003 |
| 17b | 17c | 0.009 |
| 17a | 17c | 0.27 |
| 23a | 23b | 0.78 |
Table 3. The Mann-Whitney test results: pairwise comparison of TIA in Hedysarum gmelinii plants in different years of observation in the same habitats.
High TIA values were recorded in the populations from central area of AR (5, 16, 17, 19), but sometimes with annual fluctuations. For example, the average TIA value in individuals from habitat No. 17 in 2011 was low (Cluster 2), and in 2012, it was 1.5 times higher (Cluster 1) (P=0.000-0.009<0.05) (Table 3). This can be explained by a significant difference in climatic conditions of 2011 vs. 2012 growing seasons. The year 2011 was characterized by unusual softness: warmer summer and sufficient precipitation, which differed from the long-term average climatic parameters (Report on… 2012a). On the contrary, in 2012, there was insufficient precipitation at the beginning of the growing season in the central part of the Altai Republic. Spring has arrived at an abnormally early date. The transition through 00С took place 5-14 days earlier, and in May abnormally sharp changes in daily temperatures were recorded: in the daytime up to+(25-30)°С, with a night drop to -(6-9)°С. In June 2012, the onset of abnormally hot weather was noted, up to+(31-36)°C, which is 3-4°C higher than the norm (Report on… 2012b). Thus, in 2012 the weather conditions in central area of AR were unfavorable and characterized by sharp fluctuations in temperature at the beginning of the growing season. It can be assumed that the high background TIA values in individuals from habitats No 5, 16, 17, 19 in 2012 resulted as a response to extreme weather conditions, and were caused by abnormally sharp temperature changes and insufficient precipitation at the beginning of the growing season. In one of the edge habitats (No. 23), during two years of study, TIA in individuals of H. gmelinii was characterized only by high values (Fig. 2a). It is difficult to interpret the results of the TIA study in habitats No 11, 14, 15 due to the lack of reliable meteorological information until 2009 in the areas where our work was carried out.
Representatives from 13 CPs were assigned to Cluster 2 (n=200). They were characterized by significantly lower mean TIA values (18.1 ± 0.7 mg/g) (P=0.0000<0.05) (Fig. 2b). The average value for a sample from the populations of this cluster is substantially lower (1.7 times) than that of individuals from Cluster 1, and the difference is statistically significant. Individuals from Cluster 2 differed from representatives of Cluster 1 by low average (less than 25 mg/g) and minimum (less than 10 mg/g) TIA values. The average TIA values for samples from this cluster varied within wider range, compared to the values for this trait in representatives of Cluster 1 (Table 2). In representatives from this cluster, the ratio of the extrema values of TIA (max/min) is significantly higher than the ratio of these values in individuals of Cluster 1 (6.4 ± 1.3); (P=0.001<0.05). We have detected a large variation in TIA values in the representatives from this Сluster (Cv=53.9%). For samples from the CP, it reached high and very high values (Tables 4 and 5). Only in AR, individuals from this cluster were growing under conditions of less continental climate in the range of absolute altitudes of 400-1100 m (Table 1).
| Habitat numbers | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5b | 11 | 14 | 15 | 16b | 17a | 17c | 18 | 19 | 23a | 23b | 24 | 25 | |
| M* | 26.2 | 32.6 | 34.3 | 23.7 | 35.5 | 37.4 | 26.7 | 33.1 | 33.7 | 30.2 | 27.6 | 35.7 | 34.6 | 24.2 | 23.2 | 21.7 | 27.0 |
| m Cv | 1.9 | 2.6 | 1.1 | 3.1 | 1.3 | 0.9 | 2.1 | 4.7 | 1.1 | 1.8 | 2.0 | 0.7 | 1.3 | 3.5 | 1.6 | 1.5 | 1.5 |
| % | 33.0 | 25.6 | 10.5 | 40.8 | 14.2 | 11.0 | 23.8 | 42.3 | 10.1 | 18.6 | 23.2 | 6.6 | 14.3 | 48.4 | 23.9 | 20.9 | 23.6 |
| Max/min | 4.4 | 2.5 | 1.5 | 3.4 | 2.1 | 1.6 | 2.0 | 3.1 | 1.4 | 1.9 | 2.3 | 1.2 | 1.6 | 6.3 | 2.4 | 2.4 | 2.4 |
Table 4. TIA (mg/g dry weight) in representatives of Hedysarum gmelinii (Cluster 1).
| S.No* | Habitat numbers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pl. | 5a | 6 | 7 | 8 | 9 | 10 | 12 | 13 | 16a | 17b | 20 | 21 | 22 |
| M** | 19.0 | 19.0 | 12.2 | 20.3 | 20.8 | 25.2 | 23.7 | 17.9 | 22.3 | 17.0 | 16.4 | 6.0 | 2.8 |
| m | 9.6 | 7.5 | 3.8 | 4.6 | 12.5 | 11.7 | 11.3 | 7.4 | 8.7 | 7.7 | 8.6 | 3.2 | 1.6 |
| Cv,% | 50.8 | 39.7 | 31.1 | 22.7 | 59.9 | 46.3 | 47.5 | 41.6 | 39.1 | 45.4 | 52.2 | 54.2 | 56.2 |
| max/min | 20.4 | 3.3 | 6.0 | 2.6 | 4.6 | 4.2 | 6.3 | 7.3 | 5.4 | 4.8 | 5.5 | - | 6.6 |
Table 5. TIA (mg/g dry weight) in representatives of Hedysarum gmelinii (Cluster 2).
Discussion
According to the literature, in mountainous areas, as a rule, a high amplitude of temperature fluctuations (from-400 to+400°C) and a hot summer growing season are observed; and plants require high frost resistance, despite of hot summers. The temperature at sunrise can be about +4°C, and consequently can reach +34°C in the afternoon on a sunny slope in summer (Larcher 2012). Therefore, local plant species must withstand both high and low temperatures (Lütz et al. 2010). In general, the climate of the mountains of Southern Siberia is rather severe for the latitudes within which they are located. Average annual temperatures are negative almost everywhere. This is due to the long duration and low temperatures of the cold season. At an altitude of 1500-2000 m, the duration of the frost-free period does not exceed 20-30 days, and frosts in any month are possible (Gvozdeckij and Mihajlov 1987). In the northern low-mountainous part of the area (south of the Krasnoyarsk Territory and Republic of Khakassia) the climate is characterized as sharply continental (Polikarpov 1986). In Western Altai, a comparatively wetter and warmer climate is observed. In central area of AR, the climate has transitional properties from the northwestern to southeastern regions of Altai. Moving from the North-West to the South-East, the conditions in the Altai mountains become more severe: moisture and heat supply decreases, and the climate continentality increases (Makunina 2016).
Adaptation of H. gmelinii in the mountains of Southern Siberia is the result of evolution, which took place over many generations. It included structural adaptation (shortening of the shoot length) and modifications of the activity of various immunity factors, one of which is TIA. In most of the studied CPs of H. gmelinii, TIA was characterized by high background values in mountain conditions. Though these conditions are considered "harsh", they are not always stressful for adapted plants (Körner 2016). In H. gmelinii, we identified one of the ways of adaptation: it is a tendency of increasing TIA values in habitats close to the boundaries of the central part of the range and in high mountains. In mid-mountain conditions (Central Altai), the species has the ability to reversibly change its TIA. In years with difficult weather conditions (sharp fluctuations of temperatures at the beginning of the growing season), the TIA values were increased. Such changes may be called "physiological adjustments" or "acclimatization". Maintaining high activity of this group of substances is energetically expensive (Zavala et al. 2004). Therefore, the level of TIA in the aerial part can serve as a criterion for assessing the state of plant immunity.
In our previous studies, we have shown bursts of IT activity (more than 100 mg/g dry weight) in leaves of Hedysarum theinum Krasnob. in culture, when plants were exposed to abnormally sharp temperature changes at the beginning of the growing season. High TIA in this case persisted throughout the growing season. In years with average long-term weather conditions, the values of TIA in these plants were significantly lower (25-30 mg/g) (Zhmud et al.2012). Our studies of H. gmelinii confirmed this trend. Under the influence of sharp temperature changes, there is a significant increase of TIA in the leaves of this species in natural populations. Similar adaptive mechanisms were found in the experimental studies of cultivated plants. It has been shown that in response to the effects of low temperature, TIA increased in the leaves of resistant potato and wheat genotypes (Ibragimov et al. 2012, Frolova et al. 2011). These experimental discoveries with cultivated plants are in support of our conclusions.
Conclusion
Our studies have shown high variability of TIA values in leaves in H. gmelinii in mountain conditions. The ability to increase activity of one of the immunity factors in extreme conditions and near the boundaries of the part of the range in H. gmelinii has developed in a long evolutionary timespan. Since similar adaptive mechanisms were found in experiments with cultivated plants, it is reasonable to assume that the ability to increase TIA values in leaves of H. gmelinii contributes to the resistance of this species in various ecological and geographical conditions of the mountains of Southern Siberia.
Acknowledgments
The work was carried out with financial support of the budgetary project of Central Siberian Botanical Garden, SB RAS "Analysis of biodiversity, conservation and restoration of rare and resource plant species by experimental methods" No AAAA-A21-121011290025-2.
References
Anderson, M.J. (2001). A new method for non‐parametric multivariate analysis of variance. Austral Ecology26:32-46.
Chye, M.L., Sin, S.F., Xu, Z.F. (2006). Serine proteinase inhibitor proteins: Exogenous and endogenous functions. In Vitro Cellular and Developmental Biology-Plant,42:100-108.
Domash, V.I., Sharpio, T.P., Zabrejko, S.A., Sosnovskaja, T.F. (2008). Proteolytic enzymes and trypsin inhibitors of higher plants under stress. Russian Journal of Bioorganic Chemistry34:353-357 (In Russian).
Dorogina, O.V., Zhmud, E.V. (2010). Trypsin inhibitor activity in leaves of fodder Leguminous plants. Siberian Journal of Agricultural Science, 10:23-28 (In Russian).
Dyson, K. (2018). Custom community ecology helper R scripts.
Dyson. K. (2019). Vegetation communities on commercial developments are heterogenous and determined by development and landscaping decisions, not socioeconomics.PloS one, 14:e0222069.
Ermakov, A.I. (1987). Methods of biochemical research of plants. Leningrad, pp:44-45 (In Russian).
Frolova, S.A., Titov, A.F., Talanova, V.V. (2011). Effect of low-temperature hardening on activities of proteolytic enzymes and their inhibitors in the leaves of wheat and cucumber seedlings. Russian Journal of Plant Physiology,58:208-212. (In Russian).
Gitlin-Domagalska, A., Maciejewska, A., Dębowski, D. (2020). Bowman-birk inhibitors: insights into family of multifunctional proteins and peptides with potential therapeutical applications. Pharmaceuticals (Basel), 13:421.
Gofman, J.J., Vajsblaj, I.M. (1975). Determination of trypsin inhibitor in pea seeds. Applied Biochemistry and Microbiology, 11:777-783 (In Russian).
Gvozdetskii, N.A., Mikhailov, N.I. (1987). Physical geography of the USSR. Asian part. Textbook for students of geographical specialties of universities. Moscow. Higher School Publishing House (In Russian).
Hellinger, R., Gruber, C.W. (2019). Peptide-based protease inhibitors from plants.Drug Discovery Today, 24:1877-1889.
Ibragimov, R.I., Shpirnaya, I.A., Tsvetkov, V.O., Basyrova, A.M. (2012). Activity of inhibitors of exogenous hydrolases in potato tubers at infection of phytophtora. In: Adaptive Strategies of living systems. Proceedings of the Interdisciplinary Scientific Conference,Novy Svet, AR Crimea, Ukraine, p:44 (In Russian).
Ilina, V.N., Abramova, L.M., Mustafina, A.M. (2020). The structure and state of coenopopulations of the rare species Hedysarum gmelinii Ledeb. (Fabaceae) in different parts of the rang. Samara Journal of Science, 9:42-49 (In Russian).
Kamble, V.S., Jadhav, V.D. (2016). Polyphenol content of some traditional leafy vegetables in Kolhapur district of Maharashtra. Journal of Pharmaceutical and Medicinal Research,2:66-67.
Khandaker, L., Akond, A.S., Oba, S. (2009). Air temperature and sunlight intensity of different growing period affects the biomass, leaf color and betacyanin pigment accumulations in red amaranth (Amaranthus tricolor L.). Journal Central European Agriculture10:439-448.
Kidrič, M., Kos, J., Sabotič, J. (2014). Proteases and their endogenous inhibitors in the plant response to abiotic stress. Botanica Serbica,38:139-158.
Körner, C. (2016). Plant adaptation to cold climates. Version 1. F1000Res. 5: F1000 Faculty Rev-2769.
Kumar, S., Kumar, S.V. (2017). Plant adaptation in mountain ecosystem. Plant Biotechnology: Principles and Applications, pp:249-271.
Larcher, W. (2012). Bioclimatic temperatures in the High Alps. In: Lütz C, editor. Plants in alpine regions. Springer, Wien, pp:21-28.
Larcher, W., Kainmuller, C., Wagner, J. (2010). Survival types of high mountain plants under extreme temperatures. Flora: Morphology, Distribution, Functional Ecology of Plants, 205:3-18.
López, Y.N., Labra, J., Délano-Frier, E.P., Barrios, A.B. (2004). Light intensity and activity of trypsin inhibitors in amaranth leaves and seeds. Artículo Científico Revista Fitotecnia Mexicana, 27:127-132.
Lütz, J.A., Wagtendonk, J.W., Franklin, J.F. (2010). Climatic water deficit, tree species ranges, and climate change in Yosemine National Park. Journal of Biogeography,37:936-950.
Madamanchi, N.R., Kus, J. (1991). Indused systemic resistance in plants In: Cole GT, Hoch HC, editors. The fungal spore and disease initiation in plants and animals.New York. Plenum Press, pp:347-362.
Makunina, N.I. (2012). Temperately cold forest-steppe of Altai. Turczaninowia, 15:108-124 (In Russian).
Makunina, N.I. (2016). The forest-steppe vegetation of the West Siberian plain and the Altai-Sayan mountain region. Novosibirsk: Academic Publishing House «Geo» (In Russian).
Mann, H.B., Whitney, D.R. (1947). On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics, pp:50-60.
Mirkin, B.M., Naumova, L.G. (2011). Short course in general ecology. Part 1: Ecology of species and populations. Ufa: publishing house BGPU (In Russian).
Mosolov, V.V., Valueva, T.A. (2008). Proteinase inhibitors in plant biotechnology (review). Applied Biochemistry and Microbiology 44:261-269 (In Russian).
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., O’hara, R.B., Simpson, G.L., Wagner, H. (2010). Vegan: community ecology package. R Package Version, 1:17-24.
Pjak, A.I. (2003). Petrophytes of the russian altai. Tomsk (In Russian).
Policarpov, N.P., Chebakova, N.M., Nazimova, D.I. (1986). Climate and mountain forests of Southern Siberia. Novosibirsk, publishing house «Nauka» (In Russian).
Re, G., Piluzza, G., Sanna, F., Molinu, M.G., Sulas, L. (2018). Polyphenolic composition and antioxidant capacity of legume based swards are affected by light intensity in a Mediterranean agroforestry system. Journal of the Science of Food and Agriculture, pp:191-198.
Report on the state and environmental protection of the Altai Republic. (2011). Climatic features of the year. Gorno-Altaysk, pp:70-77.
Report on the state and environmental protection of the Altai Republic. (2012). Climatic features of the year. Gorno-Altaysk, pp:42-47.
Sarker, U., Oba, S. (2018). Drought stress effects on growth, ros markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Applied Biochemistry and Biotechnology,186:1-18.
Sarker, U., Oba, S. (2018). Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chemistry,252:72-83.
Shamei, Z.E., Wu, J.W., Haard, N.F. (1996). Influence of wound injury on accumulation of proteinase inhibitors in leaf and stem tissues of two processing tomato cultivars. Journal of Food Biochemistry, 20:155-171.
Syeva, S.J., Karnauhova, N.A., Dorogina, O.V. (2008). Genus Hedysarum L. (Fabaceae Lindl.) of Altai Mountains. Gorno-Altaisk.
Trifonova, E.A., Kochetov, A.V., Shumnyj, V.K. (2007). Molecular mechanisms of systemic resistance of plants to viral infections and methods for increasing virus resistance by transgenesis. Biology Bulletin Reviews, 127:13-24 (In Russian).
Zajcev, G.N. (1984). Mathematical statistics in experimental botany. Moscow: Nauka.
Zavala, J.A., Patankar, A.G., Gase, K., Baldwin, I.T. (2004). Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuate. PNAS, 101:1607-1612.
Zhmud, E., Kuban, I., Emtseva, M., Dorogina, O. (2018). Comparative analysis of trypsin inhibitor activity in the wet and dry weight of leaves in representatives of Hedysarum L. in the foreststeppe of Western Siberia. In: IV(VI)th All-Russia Scientific-Practical BIO Web Conference “Prospects of Development and Challenges of Modern Botany”.
Zhmud, E.V. (2014). Ecological flexibility Hedysarum gmelinii (Fabaceae) in Altai Mountains and Chakassia. Tomsk State Pedagogical University Bulletin, 11:220-226.
Zhmud, E.V., Zinner, N.S., Dorogina, O.V. (2012). Dynamics of trypsin inhibitor activity in Hedysarum theinum Krasnob. (Fabaceae Lindl.) plant leaves in different ecological and geographical conditions and by mechanical damage. Tomsk State University Journal of Biology,3:100-110.
Author Info
E.V. Zhmud1*, N.B. Williams2, Y.S. Bukin3 and O.V. Dorogina12Department of Health and Exercise Science, Moby Complex, Campus 1582, Colorado State University, Fort Collins, Colorado, 80523,, USA
3Department of Physical and Chemical Biology, Limnological Institute, Faculty of Biology and Soil Studies, ISU, Ulan-Batorskaya St.3, Irkutsk, 664033, Russia
Citation: Zhmud, E.V., Williams, N.B., Bukin, Y.S., Dorogina, O.V. (2021). Specific adaptation features of Hedysarum gmelinii Ledeb. (Fabaceae Lindl.) in the mountains of South Siberia. Ukrainian Journal of Ecology 11 (9), 31-38.
Received: 05-Oct-2021 Accepted: 11-Nov-2021 Published: 20-Nov-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
