Research - (2024) Volume 14, Issue 6
Separation of phycocyanin from spirulina by photosynthetic
Man Gil Ahn*Abstract
Today, spirulina is cultivated on land in various ways. The mass production of microalgae and their bioactive compounds has been steadily increasing in response to the global demand for natural compounds. Spirulina in particular has been used due to its high nutritional value, especially its high protein content. Spirulina extracts are mainly associated with promising biological functions related to the high-value blue pigment phycocyanin. Phycocyanin is used in several industries such as food, cosmetics and pharmaceuticals, increasing its market value. Due to the global interest and the need to replace synthetic compounds with natural compounds, efforts have been made to optimize the large-scale production process and maintain the stability of phycocyanin, a highly unstable protein. This paper describes the production, extraction and purification methods, including the main physical and chemical parameters that can affect the purity, recovery and stability of phycocyanin.
Keywords
Dried, Spirulina, Phycocyanin, Photosynthesis, Water, Seawater, Pigment.
Introduction
Arthrospira platensis, known as Spirulina, is the most widely used natural resource, especially in the food industry.
Spirulina cultivation is cost-effective, easy to harvest and can thrive in a variety of conditions, such as high pH and eutrophic or mixed trophic conditions, thus minimizing the risk of contamination by other microorganisms.
Spirulina is rich in value-added compounds, mainly polyunsaturated fatty acids and pigments, especially phycocyanins.
Phycocyanins are water-soluble, non-toxic and blue photosynthetic pigments known to be used in the food, cosmetic and pharmaceutical industries.
The rate of extraction of phycocyanins from Spirulina, the efficiency of cell disruption methods and the instability of phycocyanins in both storage conditions and product formulations limit their applicability
Optimization of Spirulina culture conditions for producing phycocyanins, extraction and purification conditions to achieve high purity and stability of phycocyanins for mass production are therefore of great interest to the scientific community. Optimal Spirulina cultivation conditions for phycocyanin production, extraction and purification methods and physical and chemical conditions affecting the purity and stability of this protein are described.
Blue pigment in nature
Blue pigment in nature
Blue is a very noticeable color on Earth. But blue is very rare when it comes to nature. Less than one in ten plants has blue flowers and much fewer animals are blue. Blue flowers are rare in plants, but few have green leaves except for a few plants found at the bottom of the rainforest. The main reason for this is related to the physics of light. Pigments appear as light colors that reflect without absorbing them. The most common plant pigment is green chlorophyll, so chlorophyll does not absorb green light, but rather reflects it, so the plant looks green. However, plants like blue light because they have more energy than any other light in the visible spectrum. Animals are much more difficult to turn blue. Many pigments in animals come from the food they eat. Soflamingos are pink because of the dye they get when they eat shrimp, their favorite food and goldfish's golden color comes out as food. However, as we heard above, animals cannot turn blue through food because plants do not have true blue pigments. Blue is obtained from many animals by creating structures that change the wavelength of light instead of mixing orchanging pigments. For example, a blue moto butterfly gains color by having its wing scales shaped like a ridge so that the only wavelength the light reflects is blue. If the scales are different, the blue color disappears. The only exception in nature is the obrina olive wing butterfly, the only animal known to produce true blue pigments. Today, blue flowers are still highly regarded and many people have tried to grow and reproduce perfect blue flowers. But blue roses and carnations could still produce the first true blue chrysanthemum while avoiding us in Japan. Blue will essentially continue to be rare (Posted on August 20 2019 by Sam Le Gallou).
Pigment of marine algae
Algae contain a variety of pigments. The three main classes of pigments are chlorophyll, carotenoids (carotene and xanthophyll) and phycobilis (phycocyanin and phycoerythrin). Phycocyanin and phycoerythrin belong to the main class of phycobilins photosynthetic pigments, while fucoxanthin and peridinin belong to the carotenoid group of photosynthetic pigments. Macroalgae and microalgae (including cyanobacteria) provide a variety of metabolites, including pigments (Fig. 1).
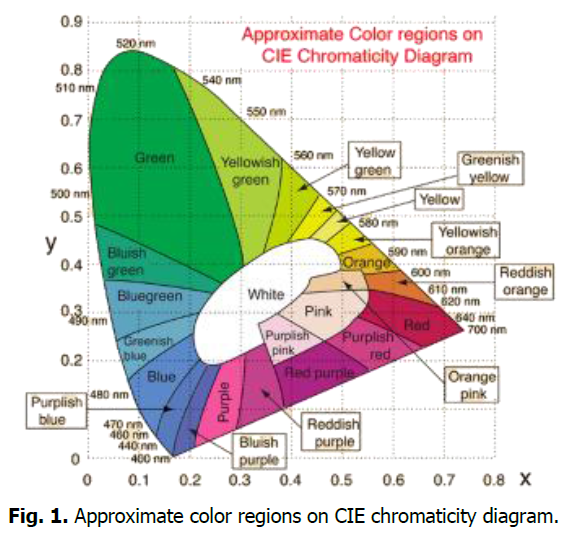
Fig. 1. Approximate color regions on CIE chromaticity diagram.
Structure of phycobiliproteins
The phycobiliproteins are antennae protein pigmentsfound in cyanobacteria, rhodophytes, cryptomonadsand cyanelles (Glazer, 1994). The phycobiliproteins are present as phycobilisomes anchored on the thylakoid membranes and lie adjacent to the photosynthetic reaction centre of the PS II in cyanobacteria and red algae. These chromoproteins are classified into 3 groups basedon the presence of different chromophores among them (Gantt, 1994; Glazer, 1985; Zilinskas, 1986; Rowan, 1989; Sidler 1994; Mac Coll, 1998; Ducret et al., 1998). These groups are (1) Phycoerythrin (PE) λmax480 nm-570 nm; (2) Phycocyanin (PC) λmax 590-630nm and Phycoerythrocyanin (PEC) λmax 630-665 nm (3) Allophycocyanin (APC) λmax 620-665 nm. Core of phycobiliproteins is composed of allophycocyanin from which arise six rods of varying length consisting of phycocyanins to the proximal side of the core and phycoerythrins to the distal side of the core.
Materials and Methods
Spirulina was as a food material in the form of dried granules and powder. The drying temperature in the cultivation and production of spirulina is usually 40-60 degrees and it survives at 70-75 degrees when placed in water. The survival test of dried spirulina conducted outdoor and the separation experiment of phycocyanin was conducted at indoor. The experiment was conducted during the day and night and the atmosphere temperature during this period ranged from a minimum of 20 degrees to 33 degrees.
Results and Discussion
Dried spirulina is alive
The concentration of seawater and water is pH 7.0-8.2 and spirulina is in an alkaline state, so when placed in water or seawater, it photosynthesizes and lives. When nutrients are added to spirulina, the cells release spores and growing and multiply (Fig. 2).

Fig. 2. Dried spirulina is sold as granules and powder for food use.
Photosynthetic spirulina reaction and separation of phycocyanin
Dried spirulina was added to 0.12-0.13 g of water and 25-28 cc of seawater and stirred. The stirred mixture was supersaturated and placed in a glass tube, where the green was separated into blue by photosynthesis. The green appeared as blue by photosynthesis and as time passed from top to bottom, the blue became saturated. The separation of blue by light reaction was fast in seawater and very slow in water. The spirulina that could not light react in a saturated state precipitated. The time required was about 9 hours in seawater and more than 15 hours in water (Fig. 3).
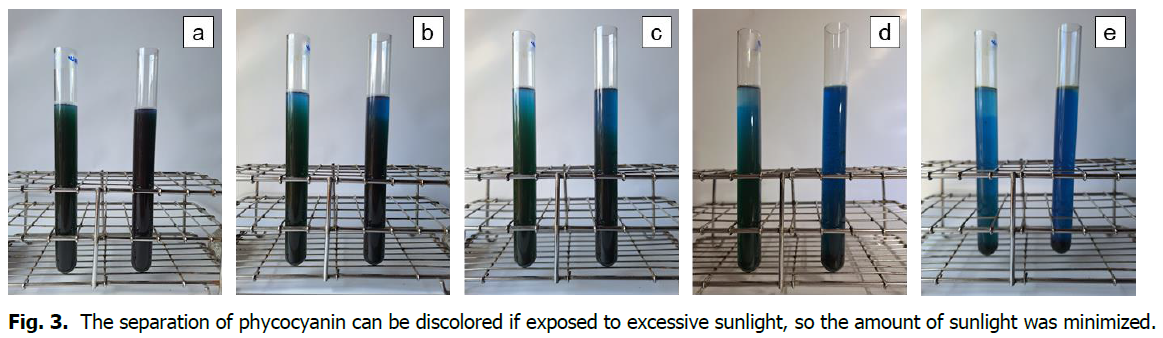
Fig. 3. The separation of phycocyanin can be discolored if exposed to excessive sunlight, so the amount of sunlight was minimized.
Spirulina in seawater
0.5 grams of spirulina was mixed with seawater and stirred, then placed in a measuring cylinder.
The seawater was divided into concentrations and the changes due to photosynthesis were confirmed. The salinity of seawater of 17.5psu reacted slowly and 27psu and 35psu were measured with the same reaction time. The sediment is the green color of spirulina. (From the left in the photo, the salinity of seawater is 17.5psu/L, 27psu/L, 35psu/L) (Fig. 4).
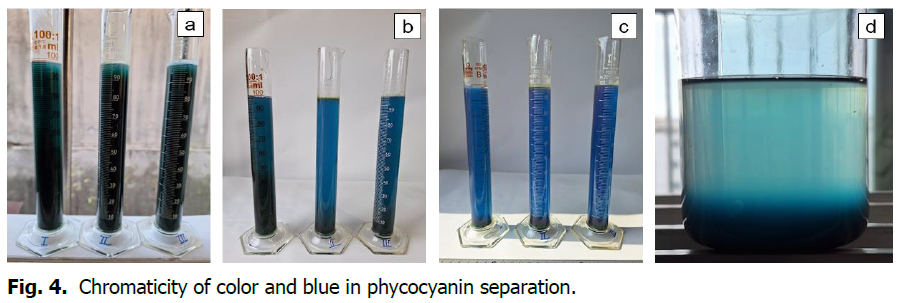
Fig. 4. Chromaticity of color and blue in phycocyanin separation.
Phycocyanin in green seaweeds of marine macro algae
Green seaweeds have nine structural colors. Photosynthetic green seaweeds produce pigments as they change color through signaling of intracellular chromosomes (Fig. 5).
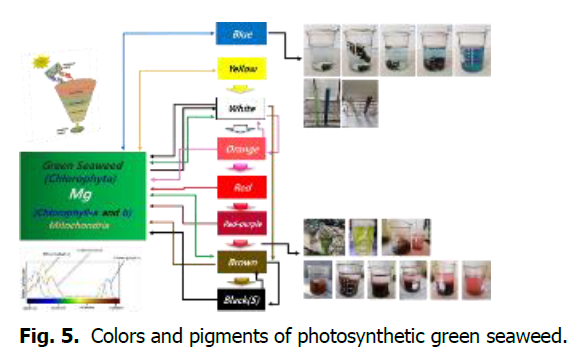
Fig. 5. Colors and pigments of photosynthetic green seaweed.
Conclusion
Phycocyanin is separated from spirulina in various ways. The process of separating pigments can cause environmental problems by consuming a lot of energy and water. Reducing the amount of energy and water consumed in the production and separation of pigments, especially by using seawater, can reduce environmental problems.
Acknowledgement
None.
Conflict of Interest
The authors declare no conflict of interest.
References
Ahn, M. G. (2021). Separation and method of natural pigments by chromaticity transformation of green seaweed. Ukrainian Journal of Ecology 11:37-46.
Ahn, M. G. (2021). “The origin of life is chemical synthesis” marine algae (green seaweed): Cellulose is a cell. Ukrainian Journal of Ecology 11:55-67.
Chandler, C. J., Wilts, B. D., Brodie, J., Vignolini, S. (2017). Structural color in marine algae. Advanced Optical Materials 5:1600646.
Google Scholar, Crossref, Indexed at
Alam, T., Najam, L., Al Harrasi, A. (2018). Extraction of natural pigments from marine algae. Journal of Agricultural & Marine Sciences 23.
Google Scholar, Crossref, Indexed at
Aryee, A. N., Agyei, D., Akanbi, T. O. (2018). Recovery and utilization of seaweed pigments in food processing. Current Opinion in Food Science 19:113-119.
Google Scholar, Crossref, Indexed at
Barkia, I., Saari, N., Manning, S. R. (2019). Microalgae for high-value products towards human health and nutrition. Marine Drugs 17:304.
Google Scholar, Crossref, Indexed at
Pangestuti, R., Kim, S. K. (2011). Neuroprotective effects of marine algae. Marine Drugs 9:803-818.
Google Scholar, Crossref, Indexed at
Suganya, T., Varman, M., Masjuki, H. H., Renganathan, S. (2016). Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renewable and Sustainable Energy Reviews 55:909-941.
Google Scholar, Crossref, Indexed at
Mouritsen, O. G., Rhatigan, P., Pérez-Lloréns, J. L. (2019). The Rise of Seaweed Gastronomy: Phycogastronomy. Botanica Marina 62:195-209.
Google Scholar, Crossref, Indexed at
González-Torres, L., Churruca, I., Schultz Moreira, A. R., Bastida, S., Benedí, J., Portillo, M. P., Sánchez-Muniz, F. J. (2012). Effects of restructured pork containing Himanthalia elongata on adipose tissue lipogenic and lipolytic enzyme expression of normo-and hypercholesterolemic rats. Journal of Nutrigenetics and Nutrigenomics 5:158-167.
Google Scholar, Crossref, Indexed at
Olivero-David, R., Schultz-Moreira, A., Vázquez-Velasco, M., González-Torres, L., Bastida, S., Benedí, J., Sánchez-Muniz, F. J. (2011). Effects of Nori-and Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipaemia and lipoproteinaemia in growing Wistar rats. British journal of nutrition, 106:1476-1486.
Google Scholar, Crossref, Indexed at
Gammone, M. A., D’Orazio, N. (2015). Anti-obesity activity of the marine carotenoid fucoxanthin. Marine Drugs 13:2196-2214.
Google Scholar, Crossref, Indexed at
Garcimartín, A., Benedí, J., Bastida, S., Sánchez-Muniz, F. J. (2015). Aqueous extracts and suspensions of restructured pork formulated with Undaria pinnatifida, Himanthalia elongata and Porphyra umbilicalis distinctly affect the in vitro α-glucosidase activity and glucose diffusion. LWT-Food Science and Technology 64:720-726.
Ali, M. Y., Kim, D. H., Seong, S. H., Kim, H. R., Jung, H. A., Choi, J. S. (2017). α-Glucosidase and protein tyrosine phosphatase 1B inhibitory activity of plastoquinones from marine brown alga Sargassum serratifolium. Marine Drugs 15:368.
Google Scholar, Crossref, Indexed at
Schultz Moreira, A. R., Olivero-David, R., Vazquez-Velasco, M., González-Torres, L., Benedi, J., Bastida, S., Sanchez-Muniz, F. J. (2014). Protective effects of sea spaghetti-enriched restructured pork against dietary cholesterol: Effects on arylesterase and lipoprotein profile and composition of growing rats. Journal of Medicinal Food, 17:921-928.
Google Scholar, Crossref, Indexed at
Maeda, H., Fukuda, S., Izumi, H., Saga, N. (2018). Anti-oxidant and fucoxanthin contents of brown alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Marine Drugs 16:255.
Google Scholar, Crossref, Indexed at
Maeda, H., Kanno, S., Kodate, M., Hosokawa, M., Miyashita, K. (2015). Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Marine Drugs 134799-4813.
Google Scholar, Crossref, Indexed at
Dong, X., Bai, Y., Xu, Z., Shi, Y., Sun, Y., Janaswamy, S., Qi, H. (2019). Phlorotannins from Undaria pinnatifida sporophyll: Extraction, antioxidant and anti-inflammatory activities. Marine Drugs 17:434.
Google Scholar, Crossref, Indexed at
Lee, D., Nishizawa, M., Shimizu, Y., Saeki, H. (2017). Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Research International 100:514-521.
Google Scholar, Crossref, Indexed at
Deethae, A., Peerapornpisal, Y., Pekkoh, J., Sangthong, P., Tragoolpua, Y. (2018). Inhibitory effect of Spirogyra spp. algal extracts against herpes simplex virus type 1 and 2 infection. Journal of Applied Microbiology 124:1441-1453.
Google Scholar, Crossref, Indexed at
Dinesh, S., Menon, T., Hanna, L. E., Suresh, V., Sathuvan, M., Manikannan, M. (2016). In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. International Journal of Biological Macromolecules 82:83-88.
Google Scholar, Crossref, Indexed at
Karpiński, T. M., & Adamczak, A. (2019). Fucoxanthin-an antibacterial carotenoid. Antioxidants 8:239.
Google Scholar, Crossref, Indexed at
Lu, W. J., Lin, H. J., Hsu, P. H., Lai, M., Chiu, J. Y., Lin, H. T. V. (2019). Brown and red seaweeds serve as potential efflux pump inhibitors for drug‐resistant Escherichia coli. Evidence‐Based Complementary and Alternative Medicine 2019:1836982.
Google Scholar, Crossref, Indexed at
Capillo, G., Savoca, S., Costa, R., Sanfilippo, M., Rizzo, C., Lo Giudice, A., Faggio, C. (2018). New insights into the culture method and antibacterial potential of Gracilaria gracilis. Marine Drugs 16:492.
Google Scholar, Crossref, Indexed at
Martin, L. J. (2015). Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Marine Drugs 13:4784-4798.
Google Scholar, Crossref, Indexed at
Wang, J., Ma, Y., Yang, J., Jin, L., Gao, Z., Xue, L., Zou, X. (2019). Fucoxanthin inhibits tumour‐related lymphangiogenesis and growth of breast cancer. Journal of Cellular and Molecular Medicine 23:2219-2229.
Google Scholar, Crossref, Indexed at
Liu, Q., Wang, Y., Cao, M., Pan, T., Yang, Y., Mao, H., Liu, G. (2015). Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. International Immunopharmacology 25:465-473.
Google Scholar, Crossref, Indexed at
Chen, Y., Lin, H., Li, Z., Mou, Q. (2015). The anti-allergic activity of polyphenol extracted from five marine algae. Journal of Ocean University of China 14:681-684.
Google Scholar, Crossref, Indexed at
Lin, J., Huang, L., Yu, J., Xiang, S., Wang, J., Zhang, J., Wang, Q. (2016). Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Marine Drugs 14:67.
Google Scholar, Crossref, Indexed at
Silva, J., Alves, C., Pinteus, S., Mendes, S., Pedrosa, R. (2018). Neuroprotective effects of seaweeds against 6-hydroxidopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complementary and Alternative Medicine 18:1-10.
Google Scholar, Crossref, Indexed at
Shimazu, T., Kuriyama, S., Hozawa, A., Ohmori, K., Sato, Y., Nakaya, N., Tsuji, I. (2007). Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. International Journal of Epidemiology 36:600-609.
Google Scholar, Crossref, Indexed at
Ikeda, K., Kitamura, A., Machida, H., Watanabe, M., Negishi, H., Hiraoka, J., Nakano, T. (2003). Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke‐prone spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology 30:44-48.
Google Scholar, Crossref, Indexed at
Azeem, M., Iqbal, N., Mir, R. A., Adeel, S., Batool, F., Khan, A. A., Gul, S. (2019). Harnessing natural colorants from algal species for fabric dyeing: A sustainable eco-friendly approach for textile processing. Journal of Applied Phycology 31:3941-3948.
Google Scholar, Crossref, Indexed at
Palabiyik, I., Durmaz, Y., Öner, B., Toker, O. S., Coksari, G., Konar, N., Tamtürk, F. (2018). Using spray-dried microalgae as a natural coloring agent in chewing gum: Effects on color, sensory and textural properties. Journal of Applied Phycology 30:1031-1039.
Google Scholar, Crossref, Indexed at
El-Khatib, E. M., Ali, N. F., El-Mohamedy, R. S. R. (2016). Enhancing dyeing of wool fibers with colorant pigment extracted from green algae. Journal of Chemical and Pharmaceutical Research 8:614-619.
Wang, H. M. D., Li, X. C., Lee, D. J., Chang, J. S. (2017). Potential biomedical applications of marine algae. Bioresource Technology 244:1407-1415.
Google Scholar, Crossref, Indexed at
Singh, S., Kate, B. N., Banerjee, U. C. (2005). Bioactive compounds from cyanobacteria and microalgae: An overview. Critical Reviews in Biotechnology 25:73-95.
Google Scholar, Crossref, Indexed at
Kim, S. K., & Pangestuti, R. (2011). Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Advances in Food and Nutrition Research 64:111-128.
Google Scholar, Crossref, Indexed at
Senge, M. O., Ryan, A. A., Letchford, K. A., MacGowan, S. A., Mielke, T. (2014). Chlorophylls, symmetry, chirality and photosynthesis. Symmetry 6:781-843.
Google Scholar, Crossref, Indexed at
Poojary, M. M., Barba, F. J., Aliakbarian, B., Donsì, F., Pataro, G., Dias, D. A., Juliano, P. (2016). Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Marine Drugs 14:214.
Google Scholar, Crossref, Indexed at
Matsuno, T. (2001). Aquatic animal carotenoids. Fisheries Science 67:771-783.
Google Scholar, Crossref, Indexed at
Glazer, A. N. (1994). Phycobiliproteins-a family of valuable, widely used fluorophores. Journal of Applied Phycology 6:105-112.
Google Scholar, Crossref, Indexed at
Bryant, D. A., Guglielmi, G., de Marsac, N. T., Castets, A. M., Cohen-Bazire, G. (1979). The structure of cyanobacterial phycobilisomes: A model. Archives of Microbiology 123:113-127.
Google Scholar, Crossref, Indexed at
Pimentel, F. B., Alves, R. C., Rodrigues, F., PP Oliveira, M. B. (2017). Macroalgae-derived ingredients for cosmetic industry-an update. Cosmetics 5:2.
Google Scholar, Crossref, Indexed at
Connan, S. (2015). Spectrophotometric assays of major compounds extracted from algae. Natural Products from Marine Algae: Methods and Protocols 75-101.
Google Scholar, Crossref, Indexed at
Fabrowska, J., Messyasz, B., Szyling, J., Walkowiak, J., Łęska, B. (2018). Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycological Research, 66:52-57.
Google Scholar, Crossref, Indexed at
Alam, T., Najam, L., Al Harrasi, A. (2018). Extraction of natural pigments from marine algae. Journal of Agricultural & Marine Sciences 23.
Google Scholar, Crossref, Indexed at
Wiltshire, K. H., Boersma, M., Möller, A., Buhtz, H. (2000). Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquatic Ecology 34:119-126.
Google Scholar, Crossref, Indexed at
Inskeep, W. P., Bloom, P. R. (1985). Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiology 77:483-485.
Google Scholar, Crossref, Indexed at
Ritchie, R. J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynthesis Research 89:27-41.
Google Scholar, Crossref, Indexed at
Ritchie, R. J. (2008). Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol or ethanol solvents. Photosynthetica 46:115-126.
Google Scholar, Crossref, Indexed at
Seely, G. R., Duncan, M. J., Vidaver, W. E. (1972). Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Marine Biology 12:184-188.
Google Scholar, Crossref, Indexed at
Beer, S., Eshel, A. (1985). Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Australian Journal of Marine and Freshwater Research 36:785-792.
Google Scholar, Crossref, Indexed at
Lawrenz, E., Fedewa, E. J., Richardson, T. L. (2011). Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. Journal of Applied Phycology 23:865-871.
Google Scholar, Crossref, Indexed at
Munier, M., Jubeau, S., Wijaya, A., Morancais, M., Dumay, J., Marchal, L., Fleurence, J. (2014). Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chemistry 150:400-407.
Google Scholar, Crossref, Indexed at
Hussein, M., El-Naggar, N., El-Sawah, A. (2017). Extraction, purification and spectroscopic characterization of phycobiliproteins extracted from some Nostoc Spp. Journal of Agricultural Chemistry and Biotechnology 8:261-264.
Google Scholar, Crossref, Indexed at
Thoisen, C., Hansen, B. W., Nielsen, S. L. (2017). A simple and fast method for extraction and quantification of cryptophyte phycoerythrin. MethodsX 4:209-213.
Google Scholar, Crossref, Indexed at
Pereira, T., Barroso, S., Mendes, S., Amaral, R. A., Dias, J. R., Baptista, T., Gil, M. M. (2020). Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chemistry 321:126688.
Google Scholar, Crossref, Indexed at
Floc’h, J. Y., Pajot, R., Mouret, V. (1996). Undaria pinnatifida (Laminariales, Phaeophyta) 12 years after its introduction into the Atlantic Ocean. Springer Netherlands 217-222.
Google Scholar, Crossref, Indexed at
Veiga, P., Torres, A. C., Rubal, M., Troncoso, J., Sousa-Pinto, I. (2014). The invasive kelp Undaria pinnatifida (Laminariales, Ochrophyta) along the north coast of Portugal: Distribution model vs. field observations. Marine Pollution Bulletin 84:363-365.
Google Scholar, Crossref, Indexed at
Rezzoum, N., Mouradi, A., Givernaud, T., Bennasser, L. (2017). Temporal variation of Laminaria ochroleuca Bachelot de la Pylaie (Laminariales, Phaeophyceae) biomass on the Moroccan Atlantic coast: Implication for commercial harvesting. Algological Studies 1-15.
Google Scholar, Crossref, Indexed at
Pimentel, F. B., Cermeño, M., Kleekayai, T., Harnedy, P. A., FitzGerald, R. J., Alves, R. C., Oliveira, M. B. P. (2020). Effect of in vitro simulated gastrointestinal digestion on the antioxidant activity of the red seaweed Porphyra dioica. Food Research International 136:109309.
Google Scholar, Crossref, Indexed at
Soni, R. A., Sudhakar, K., Rana, R. S. (2019). Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Reports 5:327-336.
Google Scholar, Crossref, Indexed at
Syad, A. N., Shunmugiah, K. P., Kasi, P. D. (2013). Seaweeds as nutritional supplements: Analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomedicine & Preventive Nutrition 3:139-144.
Google Scholar, Crossref, Indexed at
Sartory, D. P., Grobbelaar, J. U. (1984). Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177-187.
Google Scholar, Crossref, Indexed at
Dasgupta, C. N. (2015). Algae as a source of phycocyanin and other industrially important pigments. Algal Biorefinery: An Integrated Approach 253-276.
Google Scholar, Crossref, Indexed at
Pither, R. J. (2003). Canning| Quality changes during canning.
Brereton, R. G., Rahmani, A., Liang, Y. Z., Kvalheim, O. M. (1994). Investigation of the allomerization reaction of chlorophyll a: Use of diode array HPLC, mass spectrometry and chemometric factor analysis for the detection of early products. Photochemistry and Photobiology 59:99-110.
Google Scholar, Crossref, Indexed at
Yoshikawa, H., Hirano, A., Arakawa, T., Shiraki, K. (2012). Mechanistic insights into protein precipitation by alcohol. International Journal of Biological Macromolecules, 50:865-871.
Google Scholar, Crossref, Indexed at
Schilcher, G., Schlagenhauf, A., Schneditz, D., Scharnagl, H., Ribitsch, W., Krause, R., Horina, J. H. (2013). Ethanol causes protein precipitation-new safety issues for catheter locking techniques. PLoS One 8:e84869.
Google Scholar, Crossref, Indexed at
Pereira, L., Neto, J. M. (Eds.). (2014). Marine algae: Biodiversity, taxonomy, environmental assessment and biotechnology. CRC Press.
Takaichi, S. (2011). Carotenoids in algae: Distributions, biosyntheses and functions. Marine Drugs 9:1101-1118.
Google Scholar, Crossref, Indexed at
Guedes, A. C., Amaro, H. M., Malcata, F. X. (2011). Microalgae as sources of carotenoids. Marine Drugs 9625-644.
Google Scholar, Crossref, Indexed at
Lalegerie, F., Gager, L., Stiger-Pouvreau, V., Connan, S. (2020). The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. Academic Press 95:247-287.
Google Scholar, Crossref, Indexed at
Lordan, S., Ross, R. P., Stanton, C. (2011). Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Marine Drugs 9:1056-1100.
Google Scholar, Crossref, Indexed at
Zerrifi, S. E. A., El Khalloufi, F., Oudra, B., Vasconcelos, V. (2018). Seaweed bioactive compounds against pathogens and microalgae: Potential uses on pharmacology and harmful algae bloom control. Marine Drugs 16:55.
Google Scholar, Crossref, Indexed at
Author Info
Man Gil Ahn*Citation: Ahn, MG. (2024). Separation of phycocyanin from spirulina by photosynthetic. Ukrainian Journal of Ecology. 14:9-15.
Received: 02-Nov-2024, Manuscript No. UJE-24-153035; , Pre QC No. P-153035; Editor assigned: 04-Nov-2024, Pre QC No. P-153035; Reviewed: 18-Nov-2024, QC No. Q-153035; Revised: 23-Nov-2024, Manuscript No. R-153035; Published: 30-Nov-2024, DOI: 10.15421/2024_583
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
