Research - (2020) Volume 10, Issue 1
Abstract
The biological properties of essential oils, including pest control, antibacterial and antifungal oils, are currently the subject of a great deal of research around the world to meet the requirements of organic farming, such as the development of biopesticides based on natural plant molecules and effective against thebioaggressors in crops and stored commodities. Fungicide control is mainly based o n the use of chemical pesticides which, unfortunately, lose their effectiveness in the face of pathogenic microorganisms that develop more resistance against them. This chemical control thus becomes ineffective, expensive and dangerous for humans, their agricultur al products and the environment.The problems of the resistance and harmfulness of these synthetic products have led to the need t o find alternatives as effective but healthier. Thus, to reduce the use of chemical pesticides and enhance the value of Algerian flora, we are interested in a marginalized plant species, namely the Nettle (Urtica dioica L.) of the family Urticaceae. Indeed, the extracts of certain plants such as Medicinal and Aromatic Plants "MAP" and their constituents have long been recognized as antimicrobial agents, yet their use in pest control of crops has been very little reported. In this perspective, this study focuses on obtaining aqueous extracts, methalonic and ethanolic plant selects of their effectiveness in the fight against pests crops (especially fungi). In addition, the efficacy tests were conducted as a fungicide on Aspergillus and Fusarium. Our results were very remarkable for both efficacy tests. In fact, the inhibition of the fungi tested was proportional to the concentration applied. In conclusion, we can say that the yield of aqueous extract obtained from the leaves of the tested plant is interesting and its inhibitory effects indicate a certain effect in biological control against the bio-aggressors of the crops and stored foodstuffs.
Keywords
Organic aggressors; Fungi; Urtica dioica; Crops; Stored commodities
Introduction
In the world, between 5 and 15% of the total weight of cereals and legumes is lost each year after harvest (Hill,1990). Cereal production (wheat, barley, etc.) must be stored because it is carried out by a single annual harvest while the consumption period is extended throughout the year. Microorganisms such as mold from crops and stored foods that reduce yields and devalue nutritional value, alter organoleptic appearance and cause economic problems due to grain detoxification costs.
Due to their efficiency and easy and practical application, the use of chemical pesticides (fungicides, insecticides, etc.) is one of the components of the fabulous increase in yields observed in recent decades, especially in intensive agriculture. However, despite their effectiveness, the excessive use of these products is currently a problem and their impact has certainly been insufficiently estimated, as the most direct consequences include the depletion of useful auxiliary fauna, leading to serious disturbances in bioceanic equilibrium, the emergence of resistance, and finally environmental contamination with the appearance of toxic residues in harvested foodstuffs or their processing products. Finally, they are expensive and dangerous for human health and animals.
Indeed, in plant protection, control of fungal and bactericide is mainly based on the use of chemical pesticides, which, unfortunately, lose their effectiveness in the face of bio-aggressors or pests that develop more resistance to them as a result of their repeated application (Lamberth et al., 2013). In addition, stored foods also suffer losses of between 5 and 15 per cent of the total weight of grains, legumes and oilseeds worldwide (Hill, 1990), due to the proliferation of many deterioration agents such as rodents and insects, as well as microorganisms such as bacteria and mold (Mishra and Dubey, 1994).
Thus, these problems of resistance and harmfulness of synthetic pesticides have led to the need for more effective and healthier alternatives (El Idrissi et al., 2014) and in this context plants have, by natural selection during their evolution, developed mechanisms for adapting to the various environmental conditions, in particular the bioactive natural substances (Zeghad, 2009) which make up secondary metabolism (Deshayes, 1991; Mohammedi, 2006). Recent discoveries of antimicrobial activities, essential oils, are currently a very important database for the rigorous scientific development inherent in biological control through the use of these natural substances. To this end, we tested and evaluated antifungal activity of ethanolitic, methanolitic, and aqueous extracts prepared from leaves and stems of Nettle (Urtica dioica L.), (Quezel and Santa, 1963) to develop new natural bioactive products in place of chemical pesticides to protect crops and preserve human health and the environment.
Materials and Methods
Plant materials
Nettle (Urtica dioica L.) is a herbaceous perennial that has been used for centuries in folk medicine. The Genus Urtica belongs to family Urticaceae with about 80 species throughout the world. genus Urtica, species dioica, (Latin uro or urere="the one that burns", dioica comes from dioic=male and female flowers on separate feet), (Delahaye, 2015). Urticaceae with hairs (genus Urtica) or without hairs (genera Parietaria and Boehmeria) are distinguished (APGII, 2003ïÂÂÂÂ. The plant has been reported to have various pharmacological activities such as antioxidant, antibacterial, antimicrobial, antifungal (Gulcin et al., 2004; Hadizadeh et al., 2009) (Figure 1).
Figure 1. Female Nettle (a) and Male Nettle (b) (Draghi, 2005).
Preparation of plant extracts
The Extraction was done according to the protocol of Laoufi, (2017) improved: 20g leaf and stem powder of the plant obtained by finely grinding with a grain grinder and straw (type FRITSCH, Germany) was macerated in distilled water (aqueous extract), 200ml methanol (methanolic extract) and 200ml ethanol (ethanolic extract) for 24 hours at room temperature and then the three extracts are retrieved using filter paper (0.5μm). Then, the filtrate is concentrated in Rotavapor (Buchi Rotavapor type R-210) at 40°C for 30 minutes to remove the solvent, allowing obtaining a dry residue that is kept in a container in the shade at 4°C until it is used.
Phytochemical analysis of Urtica dioica leaves extract
a)Chromatographic analyzes
These were performed on a Hewlett-Packard gas phase chromatograph (6890) coupled with a Mass Spectrometer (GIC/SM) at the Analytical Chemistry Laboratory, Faculty of Medicine, University of Algiers1. The apparatus is equipped with an HP-5MS hair column (30 m ï?´ 0.25 mm), with a film thickness of 0.25 μg m.Thetemperature of the column is programmed from 50°C to 250°C at 4°C. min-1. The vector gas is the helium with a flow rate of 1,5 ml. min-1. Injection mode is split mode (leakage ratio: 1/70), fragmentation is carried out by electronic impact at 70 eV. The device is connected to a computer system that manages a NIST 98 mass spectrum library and is driven by HP ChemStation software to monitor the evolution of chromatographic analyzes.
b)Fourier Transform InfraRed spectroscopy (FTIR)
This is a technique used to obtain the absorption spectrum. The spectral resolution in the number of waves per cm is equal to the reciprocal of the maximum delay (difference of step) in cm. Thus, a resolution of 4 cm-1 will be given by a delay of 0.25 cm. These spectra are made from a sample of vegetable powder of Urtica dioyca L. scattered in a powder of KBr (Potassium bromide) which are modeled in the shape of a fine and transparent pastille and then introduced into the IR spectrophotometer located at the University of Oran's Laboratory of Materials Chemistry (Algeria). IR spectra are recorded on a FTIR-8201 PC Spectrometer. The main absorption bands are given in cm-1.
Antifungal activity of plant extracts
Three fungal strains obtained from the Phytopathology Lab, Faculty of Biology, Oran University, are maintained in PDA (Potato Dextrose Agar) medium and are: Aspergillus niger (MNHN 963917), Fusarium oxysporum f. sp. lycopersici (responsible for rotting). The antifungal activity of plant extract was evaluated in vitro by the solid dilution method to determine inhibition rates. Different concentrations of plant extracts (5 and 10%) are prepared and incorporated into PDA-based culture medium. Then the mixture is poured into Petri boxes for sowing by the deposit of 5mm diameter fragments in the center of the petri dish, taken from a 7-day culture mycelial carpet. Finally, incubation occurs in darkness at 25 ± 2°C. Mycelial growth of colonies was estimated after 7 days of incubation by the average of two perpendicular diameters. The control is carried out under the same conditions, without addition of plant extracts. The rate of inhibition of mycelial growth is calculated according to Wang et al., (2006) formula:
Anti-fungal Index (1-Da/Db) × 100
(with Db=diametric growth of control and Da=diametric growth of treated fungus:
Results
Plant extract yield
The Residues of each extraction are weighed to calculate the yield that varies depending on the plant species, the organ used in the extraction, the drying conditions, the wealthy metabolite plant and the nature of the solvent used for theextraction. Thus, the yields of plant extracts obtained for the three types of extracts: Aqueous, Ethanolic and Methanolic are 12.52%, 15.57% and 17.01%, respectively. These results (Table 1) show that among the three fractions, the residual Methanolic represents the highest yield.
| Extract type | Aqueous | Ethanolic | Methanolic |
|---|---|---|---|
| Yield of the vegetable extracts (%) | 12,52% | 15.57% | 17.01% |
Table 1.The yield of the vegetable extracts of the plants used.
Figure 2.Phytochemical analysis of the leaves extact of Urtica dioica determined by Chromatography gas-Mass Spectrometry (CPG/SM).
Analysis of the chemical composition of plant extracts
Phytochemicals are chemical compounds formed during the plants normal metabolic processes. These chemicals are often referred to as secondary metabolites. The objective of this research was to Determine the chemical composition of leaves extract. The phytochemical compound screened by gas chromatography-mass spectrometry (GC-MS) method. Eight bioactive phytochemical compounds were identified in the methanolic extract of Urtica dioica. The identification of phytochemical compounds is based on the peak area and retention time molecular weight (Table 2), (Huda et al., 2015).
|
|
Reten. Time[min] |
Area [mV.s] |
Height[mV] |
Area[%] |
Height [%] |
composé |
|---|---|---|---|---|---|---|
| 1 | 3,247 | 3,009 | 0,3 | 0,8 | fumaric acid; | |
| 2 | 3,823 | 202,354 | 51,741 | 1,4 | 14,4 | gallic acid |
| 3 | 4,100 | 2,466 | 0,448 | 0,0 | 0,1 | catechins |
| 4 | 10,107 | 13700,253 | 302,394 | 96,8 | 84,0 | rutin |
| 5 | 35,750 | 8,983 | 0,373 | 0,1 | 0,1 | myricetin |
| 6 | 37,763 | 1,837 | 0,220 | 0,0 | 0,1 | quercetin; |
| 7 | 67,543 | 133,576 | 1,514 | 0,9 | 0,4 | kaempferol; |
| 8 | 97,943 | 60,872 | 0,275 | 0,4 | 0,1 | isorhamnetin. |
| Total | 14147,607 | 359,974 | 100,0 | 100,0 |
Table 2. Results of Phytochemical analysis of leaves of Urtica dioica by Chromatography gas-Mass Spectrometry (CPG/SM).
According to Figure 2 of the infra-red analysis (FTIR) of the methanolic leaf extract of Urtica dioica L., there are several links with various functions. The FTIR analysis of U. dioica leaves proved the presence of aromatic rings, alkenes, aliphatic fluoro, alcohols, ethers, carboxlic acids, esters, nitro compounds, hydrogen bonded alcohols and phenols (Figure 3).
Figure 3. Phytochemical analysis of leaves of Urtica dioica by Fourier transform infrared spectroscopy (Uncal - ORTIA -10%-G2-LE12_07_201808_44 - Detector B).
Indeed, the bands around 1100 cm-1 are assigned to the C-H link (ester function); the bands around 1600 correspond to the C=O bond (aldehyde function); the narrow bands around 2900 cm-1 correspond to the CH-link (alkene function); and finally, the wide bands around 3300 cm-1 are associated with the elongation vibration of the OH (phenol function) bond (Table 3).
| S/N | Peak (Wave number cm-Ë¡) | Intensit y | Bond | Functional group assigniment | Group frequency |
|---|---|---|---|---|---|
| 1 | 471.11 | 31,65 | Unknown | ||
| 2 | 991.31 | 22,54 | C-H | Alkenes | 675-995 |
| 3 | 1241.65 | 15,46 | Esters C-H | Alcohols, Carboxlic acids, | 1050-1300 |
| 4 | 1522.32 | 26,54 | C=O | fonction aldéhyde | 1500-1600 |
| 5 | 1588.05 | 25,14 | C=O | fonction aldéhyde | 1500-1600 |
| 6 | 1649.72 | 23,36 | C=O | fonction aldéhyde | 1500-1600 |
| 7 | 2850.55 | 21,88 | CH | fonction alcène | 2850-2970 |
| 8 | 3246.20 | 20,48 | O-H | Alcohols, Phenols | 3200-3600 |
| 9 | 3363.58 | 19,33 | O-H | Alcohols, Phenols | 3200-3600 |
| 10 | 3603.66 | 8,98 | O-H | Alcohols, Phenols | 3200-3600 |
Table 3. Results of Phytochemical analysis of leaves of Urtica dioica by Fourier transform infrared spectroscopy.
Similar results were obtained byLaoufi (2017), which noted the presence of O-H groups (3306.30 cm-1), C=C (1636.52 cm-1) and an extension of the C=O bond. Similarly, Kavtaradze et al. (2001), which found five functional clusters: 3400 cm-1 (OH), 2940 cm-1 (OCH), 28402805 cm-1 (OCH), 16301520 cm-1 (aromatic), 1253 cm-1 (furan), 280, 1235, 1035 cm-1 (lignan). The compounds which are reported from the plant are beta sitosterol, trans ferulic acid, dotriacotane, erucic acid, ursolic acid, scopoletin, rutin, quercetin and p hydroxylbenzalcohol. (Ji et al. 2007). The Table 3 shows the presence of certain phenolic acids in plant extracts such as p-coumaric acid, feruliic acid, and o-coumaric acid in frequencies between 3200 and 3600 (Wave number (cm-1). (Medic-caric et al., 2004).
Results of the fungicidal effect of the plant extracts tested.
Aromatogram is a qualitative technique that determines the sensitivity of microorganisms to a substance known as antimicrobial, in our case it is the sensitivity of fungal strains (Aspergillus niger, Fusarium oxysporum and Fusarium lycopersici) to alcoholic extracts of Nettle. This examination is such that an antibiotic is replaced by the substances to be tested. This method is based on the migratory power of these substances on solid agar medium (PDA medium =Potato Dextrose Agar). Based on the results of the antifungigram test made using the vegetable extracts of the Nettle on Aspergillus niger, Fusarium oxysporum and F. lycopersici has a remarkable fungicide effect (Table 4).
| Extract type | Aqueou s 5% | Aqueou s 10% | etha n 5% |
etha n 10% |
meth a 5% | meth a 10% |
antifongi c ARTEA | antifongi c BAYER | DMS O |
|---|---|---|---|---|---|---|---|---|---|
| fusarium oxysporu m |
16,82 | 18,95 | 16,22 | 16,98 | 17,22 | 20,25 | 43,7 | 40,32 | 6,25 |
| fusarium leucoperci si |
14,82 | 19,82 | 16,82 | 17,54 | 17,82 | 18,82 | 44,01 | 40,53 | 6,25 |
| aspergilus niger |
12,82 | 17,82 | 17,82 | 18,5 | 18,89 | 20,74 | 40,41 | 41,17 | 6,25 |
Table 4. Effect of leaves extracts of Nettle on rate of inhibition of fungal development (diameter in mm).
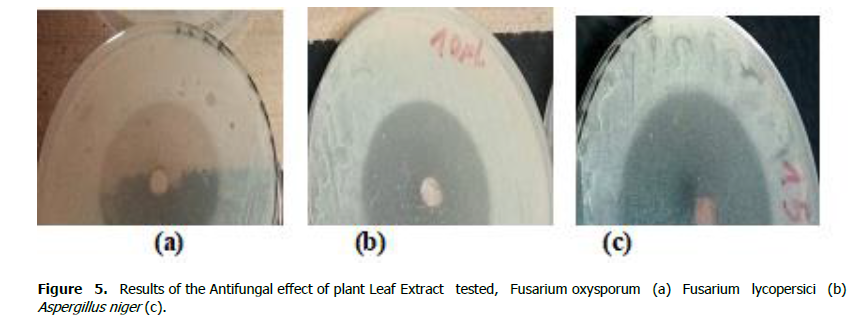
In fact, 10% aqueous extract and 10% methanolic extract are the most effective compared to the three fungi. However, they are half (20%) effective than chemical fungicides (40% to 42%), (Artea and Bayer), (Figures 4 and 5).
Figure 4.Comparison of the effect of leaf extract and synthetic antifungal on pathogen inhibition rate (diameter in mm).
Figure 5.Results of the Antifungal effect of plant Leaf Extract tested, Fusarium oxysporum (a) Fusarium lycopersici (b) Aspergillus niger (c).
Discussion
Ortie extracts are highly antimicrobial. Furthermore, the addition of samples of green plants to the storage silos of rice grain seed is a common practice of African peoples to repel certain aggressive bios from the stored commodities (Delahaye, 2015). In addition, fungal flora is a parasite of crops intended for human consumption and can have serious consequences for human health (Mishra and Dubey, 1994). This fungal development is supported by high humidity in the field and during long-term storage The dominance of the genus Aspergillus in the contaminating flora of cereals is cited in several works with Aspergillus fumigatus, the most common species followed by A. flavus and A. Niger (Pibiri, 2005).
Other strains of other genera are naturally present in crops at the plant level and in soil (Molinie and Pfohl-Leszkowczh, 2003). Thus, as a result of plant extracts, the growth of mycelial organisms is reduced or even inhibited at the concentration of the aqueous extract at 10% and the methanoic extract at the same concentration (10%) Nevertheless, they are half effective compared to the synthetic fungicides (Artea and Bayer) while the methanolic extract is more effective than the other two extracts (Figure 5). The depressive action of the secondary metabolites (Macheix and Fleuriet, 2005) of Nettle leads, in particular to a high sensitivity especially for the genus fusarium compared to the genera Aspergillus flavus and niger (Mishra and Dubey, 1994; Macheix and Fleuriet, 2005). The reducing effect of treatment with aqueous and alcoholic extracts was also reported by several authors in other species such as lavender, (Ouraïni et al., 2005). For Ncube et al., (2008), the absence of Antifungal effect on the different strains tested could be due either to the resistance of the strains or to the insufficiency of polyphenols because the plant screening and the determination of the vegetal extract showed a low phenolic compounds content for the three outputs studied, which would explain the resistance of the germs. But also, the activity of an extract is likely due to the existence of synergy between a number of components, which would become inactive individually (Rios and Recio, 2005). Similarly, the extraction method and the solvents used for extraction could be the source of these results (Hayouni et al., 2007).
Conclusion
The antimicrobial effect is likely primarily due to terpenic alcohols that are particularly active against microbial cells because soluble in aqueous media. They cause significant damage to the cell walls of microorganisms. Alcohols have fungicide effect rather than fungistatic. On the other hand, mould is still present on field seeds or on storage and the Nettle extracts used have shown a significant antifungal effect at low concentrations, but this effect varies from one plant to another. The fungistatic/fungicide tests demonstrated that the Ortie extracts have a strong antifungal effect on all mold tested. In addition, methanolic extract at the same concentration is more effective than the other two extracts than aqueous extract at 10% and ethanolic extract at 10%. Finally, the use of medicinal plants for the control of crop deterioration agents seems to us to be appropriate both for the economic interest of the operation and for its ecological interest. The use of volatile formulations based on aromatic and medicinal plants can have many advantages over current syntheses products because secondary phytometabolite formulations are neither polluting nor toxic to the environment and can have a high biocidal activity.
Acknowledgements
Thanks to the colleagues of the University of Oran, in particular the heads of Phytopathology Laboratory, Faculty of Biology, and Laboratory of Materials Chemistry University of Oran (Algeria).
References
Delahaye J. (2015). These de doctorat, Utilisations de l’ortie Urtica dioica, Université de Rouen 227.
Deshayes, A. (1991). Les transferts des capacités phytosanitaires aux plantes elles-mêmes. In: Bye, P. (Ed.), Phytosanitaires, Protection des Plantes, Biopesticides. INRA, 113-117.
Draghi F. (2005). Thèse doctorat, Ortie dioïque: étude bibliographique, Univ. Henri Poincaré Nancy; 89.
El idrissi M, Elhourri M, Amechrouq A, Boughdad A, (2014). Etude de l’activité insecticide de l’huile essentielle de Dysphania ambrosioïdes L. (Chenopodiaceae) sur Sitophilus oryzae (Coleoptera: Curculionidae) J. Mater. Environ. Sci. 5 (4), 989-994.
Gulçin, İ., Küfrevioğlu, İ., Oktay, M., M.E. Büyükokuroğlu. (2004). Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol.90: (2-3):205-215.
Hadizadeh, I., B. Peivastegan, et M. Kolahi. (2009). Antifungal activity of nettle (Urtica dioica L.), Citrullus colocynthis L. Schrad, oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pakistan Journal of Biological Sciences: PJBS 12 (1): 58-63.
Hayouni EA, Abedrabba M, Bouix M, Hamdi M, (2007). The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chemistry 105:1126-1134.
Hill, D. S. (1990). Pests of stored products and their control 274 p. CRC Press, Boca Ratón, Florida, USA,
Huda Jasim Al-Tameme, Mohammed Yahya Hadi and Imad Hadi Hameed. (2015). Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry J. Pharmacognosy Phytother Vol. 7 (10), pp. 238-252,
Ji, TF., Liu, CH., Wang, AG., Yang, JB., Su, YL., Yuan, L., (2007). Studies on the chemical constituents of Urtica dioica L. grown in Tibet Autonomous Region. Zhong Yao Cai 2007; 30:662 4
Kavtaradze, N. S., Alaniya, M. D., et Aneli, J. N. (2001). Chemical components of Urtica dioica growing in Georgia. Chemistry of natural compounds, 37 (3), 287-297.
Lamberth, C., Jeanmart, S., Luksch, T., Plant, A. (2013). Current challenges and trends in the discovery of agrochemicals. Science 341: 742–746
Laoufi, R. (2017). Caractérisation physico-chimique et biologique des extraits d’une plante médicinale algérienne de la famille des Urticaceae en vue d’une application biotechnologique. Thèse de Doctorat en biochimie- immunologie, Université M’hamed Bougara Boumerdès, Algérie, 146.
Macheix J, Fleuriet A, (2005). Les composés phénoliques des végétaux, exemple de métabolites secondaires d'importance économique, presse AURELI, presses polytechniques et universitaires romandes, 167-162.
Medic-caric, M., Jasprica, I., Smolcic-Bubalo, A., Mornar, A. (2004). Optimization of Chromatographic Conditions in Thin Layer Chromatography of Flavonoids and Phenolic Acids, Croatica Chemica Acta, CCACAA 77 (1-2) 361-366.
Mishra, A., Dubey, N. (1994). Evaluation of Some Essential Oils for Their Toxicity against Fungi Causing Deterioration of Stored Food Commodities. Applied and Environmental Microbio 60 (4), 1101-1105.
Mohammedi, Z. (2006). Etude du pouvoir antimicrobien et antioxydant des huiles essentiels et flavonoïdes de quelque plantes de la région de Tlemcen. Thèse de Magistère en Biologie option Produits Naturelles et Synthèse, Université de Tlemcen, 105p.
Molinie, A., et Pfohl-Leszkowczh, A. (2003). Les mycotoxines dans les céréales: points de contrôle de la production au stockage. Labo Toxico-sécurité alimentaire- Toulouse; N, 9.
Ouraïni, D., Agoumi, A., Ismaïli-Alaoui, M., Alaoui, K., Cherrah,Y. (2005). Study of the activity on the various stages of development of dermatophytes of essential oils from aromatic Plants with antifungal properties; Journal phytothéraopie; Paris Vol.3, N°4,147-157
Pibiri, M. C. (2005). Assainissement microbiologique de l'air et des systèmes de ventilation au moyen d'huiles essentielles. Thèse EPFL, Ecole polytechnique de lausanne. N°3311.
Quezel, P., Santa, S. (1963). Nouvelle flore d’Algérie et des régions désertiques méridionales. Tome II. Rios, J., Recio, M. (2005). Medicinal plants and antimicrobial activity. J of ethnopharmacology 100: 80-84.
Wang, J., Zhu, F., Zhou, X., Niu, C.Y., Lei, C.L. (2006). Repellent and fumigant activity of essential oil from Artemisia vulgaris to
Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Journal of Stored Products Research 42, 339-347.
Author Info
M. Sehari1, M. Kouadria1*, M. Amirat2, N. Sehari1 and A. Hassani12University of Tiaret, Veterinary Institute, Laboratory of Agro-Biotechnology and Nutrition in Semi-Arid Areas, of Tiaret, Algeria
Citation: Sehari, M., Kouadria, M., Amirat, M., Sehari, N., Hassani, A. (2019). Phytochemistry and antifungal activity of plant extracts from Nettle (Urtica dioica L.). Ukrainian Journal of Ecology, 10 (1), 1-6.
Received: 01-Jan-2020 Published: 31-Jan-2020
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.