Research - (2022) Volume 12, Issue 12
Phytochemical profile, ftir studies, antioxydant and antifungal activity of extracts of pomegranate peel (Punica granatum L)
N.H. Sehari1*, W. Siouda1, M. Sehari2, L. Merinas1 and N. Netah1Abstract
Punica granatum is an important source of bioactive drugs and has been used in folk medicine for many centuries. This paper describes the in vitro antifungal activity of pomegranate peel aqueous and alcoholic extracts against Aspergilus flavus and aspergillus niger seeds storage fungi. The yield of aqueous and alcoholic extract obtained from the peel of the tested plant is interesting and its inhibitory effects indicate a certain effect in biological control against the bio-aggressors of the crops and stored foodstuffs. This work was undertaken to valorise the different type of extracts studies of peel of pomegranate as sources of polyphenols and natural antioxidants, as well as, the FTIR spectroscopy analysis was performed to identify the function of punicalagins and ellagic acid, which are the main antifungal compounds. The screening of peel pomegranate revealed the presence of bioactive compounds: flavonoids, phenols, tannins, anthocyanins and saponosides. The methanol extracts of the plants indicated the highest values of total phenolic (16.35 ± 1.06 mg GAE/g DW), flavonoids (3.96 ± 0.56 mg QE/g DW), and tannin (7.59 ± 0.65 mg CE/g DW). The evaluation of the antioxidant activities demonstrated a strong antioxidant activity of pomegranate for all extracts tested. In vivo tests established the efficacy of treatments in controlling aspergilus flavus and aspergilus niger.
Keywords
Pomegranate peel, Antioxidant activities, Antifungal activities, Aquarius extract, Methanol extract, Activities.
Introduction
Pomegranate (Punica granatum L) or “Paradise fruit” in many ancient cultures known to human. The pomegranate is among historic native horticultural plants of Iran, which have been cultivated in different regions of the word (Ly et al., 2006). This genus comprises about 474 species. Most of the plants are annuals or perennials and grow as herbs or shrubs, diverse species of this genus take a essential role in traditional medicine and is also used in food preparation (Rdwan et al., 2006).
Plants medicinal, have for all time been a good source to discover new remedies for human health problems. Recently, a wide range of these plants have been screened for antimicrobial, fungal and insecticidal property. Punica granatum, commonly known as pomegranate, has been cited in some study as having a lots of this property (Al-Zoreky, 2009; Shreelakshmi et al., 2020).
Because of their strong astringency, Pomegranate peels are exploited in traditional medicine, what make a remedy throughout the world. In the form of an aqueous decoction (boiling the hulls in water for 10-40 minutes), it was used for dysentary and diarrhea, and also for stomatic, it can be drunk, used as a mouthwash, douche or enema (Radwan et al., 2006). Pomegranate peels a rich source of tannins, flavonoids, polyphenols and some anthocyanins, as Delphinidins, Cyanidins, etc. (Ly et al., 2006). Antioxidant and antibacterial properties of pomegranate peel in in-vitro model systems, have been reported. All the compounds of pomegranate peels are reported to have therapeutic properties (Khan et al., 2011).
The phytochemistry of pomegranate has also been widely studied by some researchers and this fruit is found to be a rich source of polyphenolic compounds (Al-Zoreky, 2009). In previous investigations was analysed the phytochemical compounds of Punica granatum peel extract and revealed that high content of ellagitannins which might be responsible for promising antioxidant and anti mutagenic activities.
Al-Zoreky, 2009, reported that pomegranate fruit peel compound punicalagin have antimicrobial activity against S. aureus and P. aeruginosa. Other works based on antifungal activity of pomegranate and some researchers. Khan et al., 2011, investigating the antifungal activities of pomegranate against Candida species reported that the fruit peel of Punica granatum L. was the most effective for inhibiting C. albicans growth. Recently published studies reported the biological efficiencies of some plant extracts against plant pathogenic fungi, and researchers explained that pomegranate Husk extracts had strong inhibitory effect against C. truncatum, C. coccodes and Rhizoctoniasolani, but this effect was not similar on Alternaria alternate and Fusarium solani. On the other hand, we encountered limited published information related to inhibition effect of pomegranate peel against Aspergillusssp. We known that some Aspergillus varieties especially A. Flavus and A. parasiticus produce a flatoxins, which are carcinogenic toxins that induce liver cancer.
The objectives of the present study were to evaluate the antioxidant and antifungal activity of hydro and methanolic extracts from peel pomegranate and to identify the chemical composition most significant by the FTIR method obtained from peel pomegranate growing in the region of benichkaw (MEDEA, center of Algeria).
Materials and Methods
Chemicals
All chemical products were purchased from biology and microbiology laboratory of the University of Yahia fares.
Plant samples
Ripe pomegranate fruits were collected in October, 2021 from pomegranate trees in medea, Algeria. Samples of ripe pomegranate fruits were handpicked from different trees of wonderful cultivar. The fruit skins were cleaned, dried in the shade and ground to fine powder. Then, two types of extracts were prepared, as described below:
The methanolic extract was prepared according to the protocol planned by Nostro and Al; 2000, with some adjustment.
50 g of pomegranate peel was mixed with 500 ml pure methanol in an Erlenmeyer flask, covered with aluminum foil, and shaken at room temperature for 24 hours. The extract was improved after filtration of the mixture with filter paper. The solvent was removed from the filtrate by vacuum evaporation using a vacuum pump in a rotavapor at 40°C. The crude extract was completely dried in a ventilated oven at 40°C.
The dried extract was stored at 4°C in amber jars until use.
Preparation of aqueous extract
Aqueous extraction was performed using the protocols adopted by Nostro and Al; 2000: 100 g of peer pomegranate were added to one liter of lukewarm distilled water and agitated by stirring for 24 hours at room temperature. After filtration, the filtrate was centrifuged at 4000 rpm for 15 min, then filtrated through filter paper, and dried in an oven at a temperature below 40°C to obtain the powder form, which was kept in dark-colored glass jars, tightly closed and stored in a refrigerator at 4°C.
Quantitative characterization of extracts
Determination of total phenols
The total phenol content was determined by using a modified Folin-Ciocalteu method as described by Beretta, et al., 2005, the Folin-Ciocalteu method. The total phenolic content was determined by spectrophotometry, following the protocol, applied by Li, et al., 2008. 200 μl of the diluted extract was mixed with 1 ml of Folin-Ciocalteu reagent diluted 10 times in distilled water. After 4 min, 800 μl of 7.5% sodium carbonate solution was added and the final volume was adjusted to 3 ml with distilled water. After incubation for 2 hours at room temperature and in the dark, the absorbance was measured at 765 nm.
Gallic acid was used as a positive control. Results were expressed in milligram equivalents of Gallic acid per gram of dry matter (mg AG/g DM).
Determination of total flavonoids
The determination of flavonoids was carried out according to the colorimetric method of Quettier-Deleu 2000 described in Djeridane, et al., (2006). One ml of plant extract (1 mg/ml) was mixed with 1 ml of aluminium chloride methanolic solution (2% AlCl3). After incubation for 10 min at room temperature, the absorbance measurement was performed at 488 nm.
A blank was prepared by mixing 1 ml of extract solution with 1 ml of methanol for each extract. The concentration of flavonoids contained in the various extracts was calculated by reference to a calibration curve, using Quercetin as the standard, and the concentration has been expressed in mg Quercetin equivalent/g dry matter.
Determination of condensed tannin
According to the vanillin method described by many researchs The Condensed tannins dosing was achieved: 50 μl of each extract were added to 1500 μl of 4% vanillin/methanol solution, vigorously mixed then 750 μl of concentrated hydrochloric acid (HCl) was added, allowed to react at room temperature for 20 min. The absorbance was measured at 550 nm against a blank. Different concentrations (0 to 1000 μg/ml) prepared from a stock solution of catechin were used to draw the calibration curve and catechin content was expressed in mg catechin equivalent of dry matter (mg Eq Cat/g DM).
Fourier transform infra red spectroscopy (FTIR)
This technique is used to obtain the absorption spectrum. The spectral resolution in the number of waves per cm is equal to the reciprocal of the maximum delay (difference of step) in cm. therefore, a resolution of 4 cm-1 will be agreed by a delay of 0.25 cm. These spectra are ended from a sample of vegetable powder of peer granate. scattered in a powder of KBr (Potassium bromide) which are modeled in the shape of a fine and transparent pastille and then introduced into the IR spectrophotometer located at the University of Oran's Laboratory of Materials Chemistry (Algeria). IR spectra are recorded on a FTIR-8201 PC Spectrometer. The main absorption bands are given in cm-1.
Antioxidant activity
DPPH radical-scavenging activity were added to 0.4 ml solution of DPPH radical in methanol.
The antioxidant activity in terms of radical scavenging ability using the DPPH of the extract was assessed according to the method of Various concentrations of pomegranate peel extracts were added, at an equal volume, to solution of DPPH radical in methanol (1000 μM). The mixture was shaken and kept in the dark at room temperature for 30 min; the absorbance was recorded at 517 nm. The experiment was replicated thrice. The antiradical activity was expressed as IC50 value (mg/ml) and the inhibition of DPPH free radical in per cent was calculated as follows:
PI (%)=[(Ablank-Asample)/Ablank] × 100 ……..(1)
Where, Asample: The absorbance of the sample, Ablank: The Absorbance of the DPPH solution.
Antifungal activity of plant extracts
Two fungal strains obtained from the Phytopathology Lab, Faculty of Biology, Tiaret University, Algeria are maintained in PDA (Potato Dextrose Agar) medium and are: Aspergillus niger (MNHN 963917), aspergillus flavus (MNSH 563317). The antifungal activity of plant extract was evaluated in vitro by the solid dilution method to determine inhibition rates. Different concentrations of plant extracts (5 and 10%) are prepared and incorporated into PDA-based culture medium. Then the mixture is poured into Petri boxes for sowing by the deposit of 5 mm diameter fragments in the center of the petri dish, taken from a 7 day 2 culture mycelial carpet. Finally, incubation occurs in darkness at 25 ± 2°C. Mycelial growth of colonies was estimated after 7 days of incubation by the average of two perpendicular diameters. The control is carried out, under the same conditions, without addition of plant extracts.
The rate of inhibition of mycelial growth is calculated according to Wang and Al, (2006) formula:
Anti-fungal Index (1-Da/Db) × 100 ………(2)
Where, Db=diametric growth of control; Da=diametric growth of treated fungus.
Statistical analysis
All experiments were conducted in triplicate and the differences between treatment means were determined by Duncan’s procedure at p<0.05 using Xl stat 6. The expressed values are mean ± standard deviation of triplicate measurements.
Results and Discussion
Plant extract yield
The results (Table 1) show that among the three fractions, the residual Methanolic represents the highest yield. Thus, the yields of plant extracts obtained for the three types of extracts: Aqueous, Ethanolic and Methanolic are 10.78%, 22.26% and 37.01%, respectively. Residues of each extraction are weighed to calculate the yield that depend on the plant species, the organ used in the extraction, the drying conditions, the wealthy metabolite plant and the nature of the solvent used for the extraction.
| Extract type | Aquarious | Metanolic | Etanolic |
|---|---|---|---|
| Yield % | 10.78 | 22.26 | 37.01 |
Table 1. The yield of the vegetable extracts of the plants used.
Phytochemical characterization
Phytochemical tests were performed on the powder of peel pomegranate by using specific revealing reagents. Phytochemical screening has highlighted the existence of secondary metabolites in the plant. The detection of these chemical compounds is based on precipitation reactions with formation of insoluble and colored complexes (foam). The observed coloration produced by the usage of an appropriate reagent, the results are expressed in the Table 2.
| Peel of pomegranate | Compounds | |||||
|---|---|---|---|---|---|---|
| Flavonoids | Tannins | antocyames | Sterols et triterpene | Phenols | Saponosides | |
| + | + | + | _ | + | _ | |
Table 2. Phytochemical characterization of peel pomegranate compounds.
Total phenol, flavonoid of different pomegranate peel extracts
In this tree (3) diverse solvents with different polarity strength were used to compare the extraction efficiency of natural molecules extracted from pomegranate peel powder (Table 2). From the results, it can be seen that all plant extracts studied are rich in polyphenols but with different amounts. In this study, the methanolic extract obviously has more fractions, ethanol and water (Table 3).
| Pomegranatepeel | Total phenolic content | Total flavonoid content (mg QE/kg extract) |
|---|---|---|
| Ethanol extract | 9.00±0.08 | 3.44±0.25 |
| Methanol extract | 19.98±0.11 | 11.95±0.95 |
| Water extract | 8.11±0.56 | 7.4±0.06 |
Table 3. Total phenolics content, total flavonoids content of different pomegranate peel extracts.
In a comparative study aimed to extract total polyphenolic and flavonoid components from pomegranate leaves, peels, and seeds using water and methanol, pomegranate peel methanolic extract had higher polyphenolic (86%) and flavonoid (52%) content than water extract (54% and 21%, respectively) followed by pomegranate leaves methanolic extract and pomegranate seed methanolic extract (El falleh, and Al, 2012).
The phenolic apparatus act usually for the antioxidant activity of extracts (Gulcin and Al, 2004) The various antioxidants may be effective as strong hydrogen giving ability (or their reducing power), free radical scavenging and metal chelating activities and they could possibly interact with biological systems and act a significant role in anticancer, anti-inflammatory, and antimicrobial activity (wang and Al, 2006; Abu reidah and Al, 2013).
Therefore, our results were supported by previous results where methanol is the most preferable solvent for extraction of the majority of polyphenolic and flavonoid compounds from pomegranate samples (El falleh, and Al, 2012). The efficiencies of the solvents used for extraction of the antioxidant phenolics were in the order: methanol>ethanol>water. The reason for this order might be due to the solubility of phenolic and flavonoid compounds which are affected by the polarity of solvents. For instance, polyphenolic compounds are polar and easily get dissolved in polar solvents such as aqueous methanol and faced difficulties with non polar solvents such as ether (Basiri, 2015; El falleh, 2012; konsoula, 2016). That is why non polar solvents such as ether, ethyl acetate, and chloroform always showed the lowest extracted phytochemical fractions. These results are in agreement with the findings confirmed by previous researchers (Basiri, 2015; El falleh, 2012).
Fourier transform infrared spectroscopy (FTIR) compounds
The FTIR analysis leaves proved the presence of aromatic rings, phenols alkenes, aliphatic fluoro, alcohols, ethers, carboxlic acids, esters, nitro compounds, hydrogen bonded alcohols and (Fig. 1).
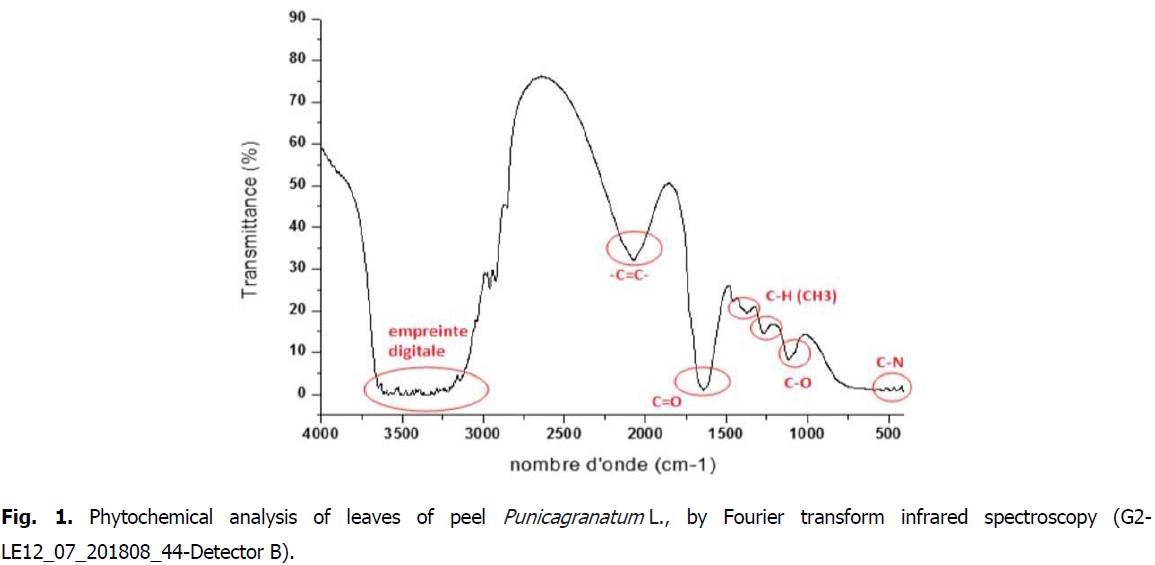
Fig 1: Phytochemical analysis of leaves of peel Punicagranatum L., by Fourier transform infrared spectroscopy (G2-LE12_07_201808_44-Detector B).
Indeed, the bands around 1100 cm-1 are assigned to the C-H link (ester function); the bands around 1600 correspond to the C=O bond (aldehyde function); the narrow bands around 2900 cm-1 correspond to the CH-link (alkene function); and finally, the wide bands around 3300 cm-1 are associated with the elongation vibration of the OH (phenol function) bond.
Phytochemical analysis of pomegranate peel powder by Fourier transform infra red spectroscopy, or FTIR spectroscopy, revealed the presence of different families of chemical compounds (Table 4.) such as phenols, (cyclic compounds) (OH Ar-OH, C6H6, C-O-C ether oxyde, C-N, C-O phenol and C=O). Indeed, according to Fig. 1, we note the presence of some phenolic acids in plant extracts such as p-coumaric acid, ferulic acid, and punicalagin most responsible of antioxidant properties of this fruit. Punicalaginis a hydrolyzable tanin and o-coumaric acid located in frequencies between 3200 and 3600, Wave number (cm-1) (Medic caric, et al., 2004).
| S.No. | Peak | Intensity (Wave number cm-1) | Bond | Functional group assignement | Group frequency |
|---|---|---|---|---|---|
| 1 | 595.85 | 3,25 | Unknown | ||
| 2 | 613.12 | 3,05 | C-H | Alkenes | 575-995 |
| 3 | 732.21 | 16,24 | C-H | Alkenes | 575-995 |
| 4 | 985.63 | 02,69 | C-H | Alkenes | 575-995 |
| 5 | 1515.45 | 10,35 | Esters C-H |
Alcohols, Carboxlic acids | 1050-1500 |
| 6 | 1634.35 | 06,50 | C=O | Functionaldéhyde | 1500-1600 |
| 7 | 2835.45 | 13,80 | CH | Functionalcène | 2850-2970 |
| 8 | 2902.22 | 09,53 | CH | Functionalcène | 2900-2970 |
| 9 | 3643.33 | 02,35 | O-H | Alcohols, Phenols | 3200-3600 |
Table 4. Results of Phytochemical analysis of pomgrenate peel by FTIR study.
Similar results were obtained by Laoufi (2017), which noted the presence of O-H groups (3306.30 cm-1), C=C (1636.52 cm-1) and an extension of the C=O bond. Also, Kavataradze, et al., (2001), they noted functional clusters: such as: 3400 cm-1 (OH) function, 2940 cm-1 (OCH) liaison, 28402805 cm-1 (OCH), between 16301520 and 1630550 cm-1 (aromatic), 1253 cm-1 (furan), 1235, 1035 cm-1 (lignan).
The results of the current study was comparable with those of Mohsen and Ammar, 2009, who shows the presence of some phenolic acids in plant extracts such as p-coumaric acid, the feruliic acid, in frequencies between 3200 and 3600 (Wave number (cm-1) and o-coumaric acid.
Antioxidant activity
The radical DPPH is generally one of the most used compounds for the rapid and direct evaluation of antioxidant activity due to its stability in radical form and the simplicity of the analysis (Gulcin, et al., 2004).
This method is based on the reduction of a methanolic solution of DPPH in the presence of an antioxidant which donates a hydrogen or an electron. Gallic acid-the method consists in comparing the absorbance of our samples with that of a calibration line which links the absorbance to the concentration.
From the results shown Fig. 2 and Fig. 3, it seems that the percentage of inhibition of the free radical increases with the increase in the concentration of the different plant extracts, therefore the anti-radical activity is proportional to the concentration of the extract.
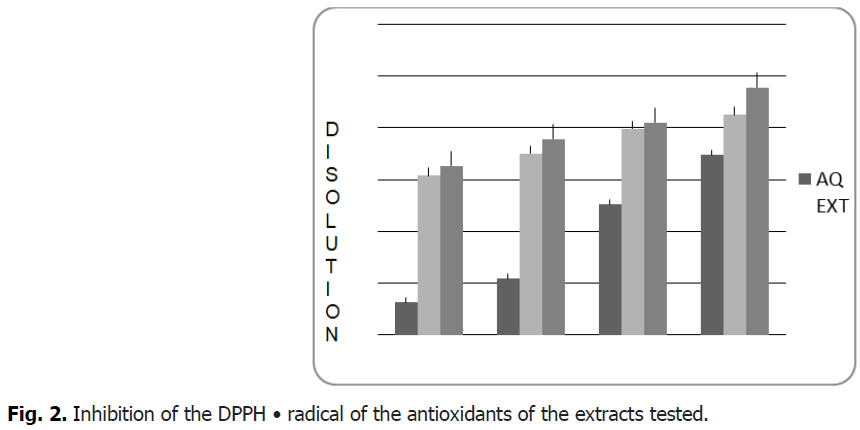
Fig 2: Inhibition of the DPPH • radical of the antioxidants of the extracts tested.
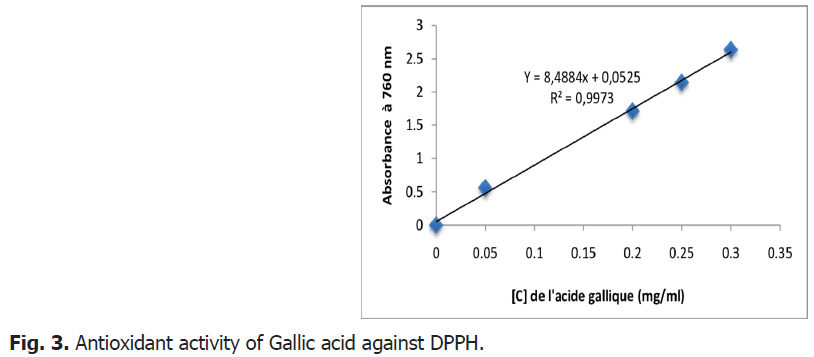
Fig 3: Antioxidant activity of Gallic acid against DPPH.
Results of the fungicidal effect of the plant extracts tested
Aromatogram is a qualitative technique that shows the sensitivity of microorganisms to a substance known as antimicrobial, in our paper the sensitivity of fungal strains (Aspergillus niger, aspergilus flavius) to alcoholic extracts of peel pomegranate. This examination is such that an antibiotic is replaced by the extract to be tested. This technique is based on the migratory power of these substances on (PDA medium=Potato Dextrose Agar). Based on the results of the antifungigram test made using the vegetable extracts of the peel on fungul tested has a remarkable fungicide effect (Fig. 4).
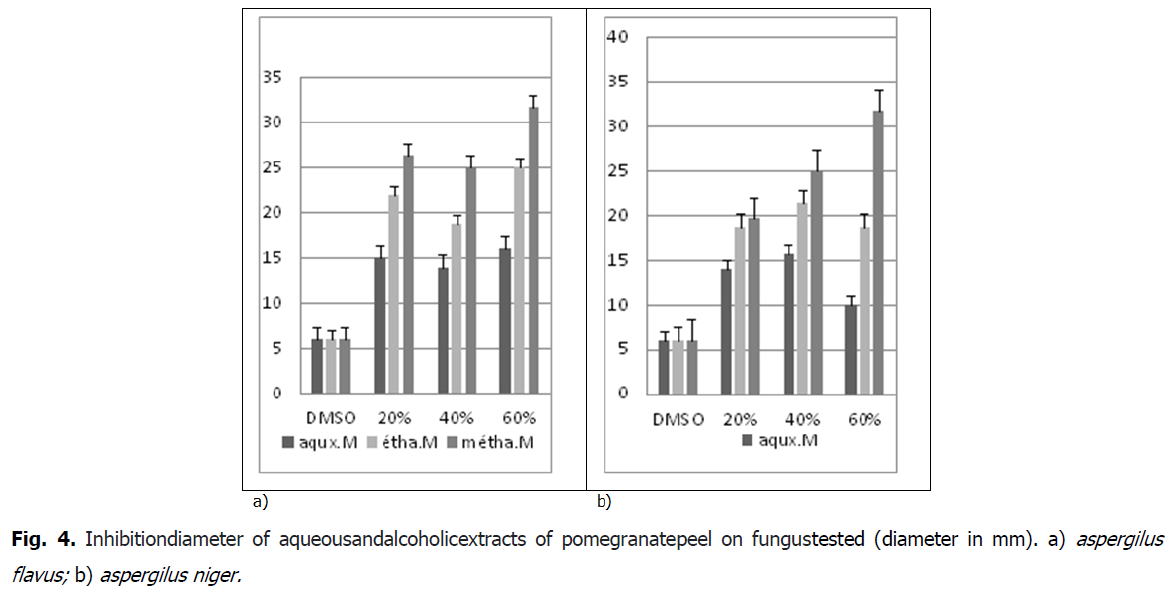
Fig 4: Inhibitiondiameter of aqueousandalcoholicextracts of pomegranatepeel on fungustested (diameter in mm). a) aspergilus flavus; b) aspergilus niger.
The methanolic extract of the peel of Punica granatum has a very strongly inhibiting antifungal effect on the fungus Aspergillus Niger for the concentrations of the extracts (20%, 40% and 60%). Approximately (Fig. 5) whith 19.33 mm to 31.66 mm, by supplying the ethanolic extract is strongly inhibitory (Fig. 5), where the growth inhibition value of the fungus reaches (18.66 mm to 21.33 mm) and aqueous gives a Moderately inhibitory effect, approximately (10 mm up to 14 mm).

Fig 5: Photography of antifungal tests of the extract (aqueous, ethanolic and methanolic) of pomegranate peel on aspergilus flavius and aspergilus niger (Original. 2022) A) Aspergilus flavus, B) 40% methanol Aspergilus niger, C) Aspergilus niger.
The fungal flora can have serious consequences for human health (Mishra and Dubey, 1994). It is a parasite of crops intended for human consumption and this fungal development is supported by high humidity in the field and during long-term storage The dominance of the genus Aspergillus in the contaminating flora of cereals is cited in several works with Aspergillus fumigatus, the most common species followed by A. flavus and A. Niger (Pibiri, 2005).
The reducing effect of treatment with aqueous and alcoholic extracts was also reported by several authors in other species such as lavender (Ourani, et al., 2005), the activity of an extract is likely due to the existence of synergy between a number of components, which would become inactive individually (Rios and Recio, 2005). Similarly, the extraction method and the solvents used for extraction could be the source of these results (Hayouni, et al., 2007).
Conclusion
In the present paper, we were interested in estimating the antioxidant and antifungal potential of the methanolic, ethanolic and aqueous extract of pomegranate peel (Punica granatum L.). The evaluation of the antioxidant activity of these extracts is unwound by two methods (scavenging of free radical DPPH and reducing power of gallic and ascorbic acid). It is noted that the punica granatum plant has a strong antioxidant activity.
The antifungal activity of the extracts was tested on 2 strains of fungi (Aspergillus flavus and Aspergillus niger) by the disk diffusion method. The zones of inhibition vary for the two fugul tested, where we notice a diameter of growth inhibition (15 ± 1.213 mm to 31.66 ± 2.415 mm) for aspergillus Flavus, we notice a similarity results with the aspergillus niger (13.18 ± 0.355 mm to 28.5 ± 1.113), these results show that the effect of pomegranate peel extracts is very strongly inhibiting for the two fungi. These results are confirmed by the statistical analyzes performed (p<0.0001)
This inhibitory potential can be used for the treatment of certain infectious diseases caused by pathogens. Extracts of, P. granatum peel may provide excellent sources of new bioactive natural products that can serve as new pharmaceutical agents to address unmet therapeutic needs.
Acknowledgement
Thanks to the colleagues of the University of Oran, in particular the heads of Phytopathology Laboratory, Faculty of Biology, and Laboratory of Materials Chemistry University of Oran (Algeria).
References
Abu-Reidah, I.M., Arráez-Román, D., Segura-Carretero, A., Fernández-Gutiérrez, A. (2013). Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chemistry, 141:2269-2277.
Google Scholar, Crossref, Indexed at
Al-Zoreky, N.S. (2009). Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology, 134:244-248.
Google Scholar, Crossref, Indexed at
Basiri, S. (2015). Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum L.) seed and defatted seed extracts. Journal of Food Science and Technology, 52:1117-1123.
Google Scholar, Crossref, Indexed at
Beretta, G., Granata, P., Ferrero, M., Orioli, M., Facino, R.M. (2005). Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Analytica Chimica Acta, 533:185-191.
Google Scholar, Crossref, Indexed at
Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., Vidal, N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry, 97:654-660.
Google Scholar, Crossref, Indexed at
Hanen, F., Riadh, K., Samia, O., Sylvain, G., Christian, M., Chedly, A. (2009). Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food and Chemical Toxicology, 47:2308-2313.
Google Scholar, Crossref, Indexed at
Gulcin, I., Şat, I.G., Beydemir, S., Elmastas, M., Küfrevioglu, O.I. (2004). Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chemistry, 87:393-400.
Google Scholar, Crossref, Indexed at
Hayouni, E.A., Abedrabba, M., Bouix, M., Hamdi, M. (2007). The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chemistry, 105:1126-1134.
Jimoh, F.O., Adedapo, A.A., Afolayan, A.J. (2010). Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food and Chemical Toxicology, 48:964-971.
Ju, E.M., Lee, S.E., Hwang, H.J., Kim, J.H. (2004). Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sciences, 74:1013-1026.
Kavtaradze, N.S., Alaniya, M.D., Aneli, J.N. (2001). Chemical components of Urtica dioica growing in Georgia. Chemistry of Natural Compounds, 3:287-287.
Google Scholar, Crossref, Indexed at
Pande, S., Sharma, M., Gaur, P.M., Tripathi, S., Kaur, L., Basandrai, A., Siddique, K.H.M. (2011). Development of screening techniques and identification of new sources of resistance to Ascochyta blight disease of chickpea. Australasian Plant Pathology, 40:149-156.
Konsoula, Z. (2016). Comparative efficacy of pomegranate juice, peel and seed extract in the stabilization of corn oil under accelerated conditions. International Journal of Nutrition and Food Engineering, 10:556-563.
Laoufi, R. (2017). Caractérisation physicochimique et biologique des extraits d'une plante médicinale algérienne de la famille des Urticaceae en vue d'une application biotechnologique.
Li, H.B., Cheng, K.W., Wong, C.C., Fan, K.W., Chen, F., Jiang, Y. (2007). Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry, 102:771-776.
Li, H.B., Wong, C.C., Cheng, K.W., Chen, F. (2008). Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Science and Technology, 41:385-390.
Medić-Šarić, M., Jasprica, I., Smolčić-Bubalo, A., Mornar, A. (2004). Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croatica Chemica Acta, 77:361-366.
Mishra, A.K., Dubey, N. (1994). Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Applied and Environmental Microbiology, 60:1101-1105.
Garcıa, V.N., Gonzalez, A., Fuentes, M., Aviles, M., Rios, M.Y., Zepeda, G., Rojas, M.G. (2003). Antifungal activities of nine traditional Mexican medicinal plants. Journal of Ethnopharmacology, 87:85-88.
Nostro, A., Germano, M.P., D’angelo, V., Marino, A., Cannatelli, M.A. (2000). Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Letters in Applied Microbiology, 30:379-384.
Ouraïni, D., Agoumi, A., Ismaïli-Alaoui, M., Alaoui, K., Cherrah, Y. (2005). Study of the activity on the various stages of development of dermatophytes of essential oils from aromatic Plants with antifungal properties. Journal Phytothéraopie, Paris, 3:147-157.
Pibiri, M.C. (2006). Assainissement microbiologique de l'air et des systèmes de ventilation au moyen d'huiles essentielles. EPFL.
Farag, R.S., El-Agaimy, M.A., Abd El Hakeem, B.S. (2010). Effects of mixing canola and palm oils with sunflower oil on the formation of trans fatty acids during frying. Food and Nutrition Sciences, 1:24.
Rios, J.L., Recio, M.C. (2005). Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology, 100:80-84.
Google Scholar, Crossref, Indexed at
Shreelakshmi, S.V., Nazareth, M.S., Kumar, S.S., Giridhar, P., Prashanth, K.V., Shetty, N.P. (2021). Physicochemical composition and characterization of bioactive compounds of mulberry (morus indica L.) fruit during ontogeny. Plant Foods for Human Nutrition, 76:304-310.
Google Scholar, Crossref, Indexed at
Wang, X.H., Aliyari, R., Li, W.X., Li, H.W., Kim, K., Carthew, R., Ding, S.W. (2006). RNA interference directs innate immunity against viruses in adult Drosophila. Science, 312:452-454.
Author Info
N.H. Sehari1*, W. Siouda1, M. Sehari2, L. Merinas1 and N. Netah12Laboratory of Agro-Biotechnology and Nutrition in Semi-Arid Areas, of Tiaret, Algeria
Citation: Sehari, N.H., Siouda, W., Sehari, M., Merinas, L., Netah, N. (2022). Phytochemical profile, ftir studies, antioxydant and antifungal activity of extracts of pomegranate peel (Punica granatum L). Ukrainian Journal of Ecology. 12:8-16.
Received: 26-Nov-2022, Manuscript No. UJE-22-81328; , Pre QC No. P-81328; Editor assigned: 29-Nov-2022, Pre QC No. P-81328; Reviewed: 14-Dec-2022, QC No. Q-81328; Revised: 20-Dec-2022, Manuscript No. R-81328; Published: 28-Dec-2022, DOI: 10.15421/2022_414
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
