Research - (2022) Volume 12, Issue 4
Phytochemical and physicochemical variations in different tea varieties and clones grown at Nthri, Shinkiari, Mansehra Pakistan
D. Kamal1*, G. Mujtabshah1, A. Waheed2, M. Abbass Khan2, N. Ahmed1, U. Jadoon1, M. Iqbal1, A. Lariab Syed1, R. Bibi1, I. Iqra1, A. Qamer1 and I. Ahmed2Abstract
Tea belongs to family of Theaceae and most popular drinking beverages of world. Present study was carried out to find out phytochemical and physicochemical analysis of different tea varieties and clone grown at PARC-National Tea and high Value Crops Research Institute, Shinkiari, Mansehra, Pakistan. Phytochemical screening was carried out using standard procedure and extract were made in three solvent i.e., methanol, ethanol and aqueous extract. Phytochemical screening of methanolic, ethanolic and aqueous extract showed the presence of tannins, Saponins, phenol, flavonoid, quniones and steroid in various tea varieties and clone. Alkaloid and anthraquniones were absent in tea variety and clone. Phlobatannins were absent in aqueous extract whereas present in all tae varieties in ethanolic extact whereas in case methanolic extact it was present in few varieties and clones. Glycosides were present in methanolic and aqueous extract whereas in ethanolic extract it was absent in some varieties of tea i.e., Turkish, Roupi, Japanese, Indonesian. Physicochemical analysis result that moisture content, ash content and crude fiber content ranges from 2.14 to 2.84, 0.09% to 0.37% and 0.08% to 0.34% respectively. Present study concluded that teas are great importance in medicine due to presence of phytochemical and good source of energy and nutrient.
Keywords
Camelliaceae, Phytochemical screening, Physicochemical analysis.
Abbreviations
NTHRI: National Tea and High Value Crops Research Institute; H2SO4: Sulphuric Acid; NaOH: SODIUM Hydroxides; HCL: Hydrochloric Acid; Kg: Kilogram; %: Percentage; Alk: Alkaloids; Fla: Flavonoids; Sap: Saponins; Cart: Carotenoids; Gly: Glycosides; Tan: Tannins; ster: steroid, ter: terpenoid ; Phlobat: Phlobatannins; anthraqun: anthraquniones; qun: quniones.
Introduction
Tea belongs to the family of Theaceae and Genus Camellia has 82 species. It grows in tropical and sub tropic regions. It is basically originated from China and drinking began in the 16th century. The Plant is medium sized, evergreen shrub. The leaves are light green, short pediculate, coriaceous, elliptical, alternate, serrate margin. Having pearl white flowers, actinomorphic, 2.5 to 4 cm in diameter, found in solitary or in clusters of two or six. Fruit is a flattened, smooth, rounded trigonous capsule, seed solitary in each, size of a small nut. It grow in tropical and subtropical region (Akhlas et al., 2003). There are three different type of tea that green, black and oblong tea which are totally depend upon chemical composition. Presently Pakistan is most second largest of tea import after U.K. The quality of Tea (Camellia sinensis. L) are best for aroma, volatile compounds and taste, colour. The value of Tea is also affected by cultural practices, environment, climatic condition, as well as tea processing techniques (Cabrera et al., 2006).
Some tea clones and varieties have been grown at NTHRI, Shinkiari on the basis of their phenotypic character and genotypic characters according to their capacity and habitat. Morphological traits and characterization is the initial steps of classification and to identify characterization for any crop. (Waheed et al., 2017). Tea are great important in medicine due to the presence of phytochemical activities. Phytochemical are word that originate from a Greek word it meaning plant chemical. It considered as uncontaminated active compound that occur biological and present in the plant which is beneficial for human health. Those bioactive compound which are present naturally in plant are called phytochemical. These phytochemical are present universally in all medicinal plant and divided into two type that is primary and secondary metabolites (Wadood et al., 2013). Chemically tea is composed of caffeine, polysaccharide, polyphenols, amino acids, sugar, alkaloids, minerals, volatile acids, trace elements and the essential oil. The quality of tea such as colour, aroma and flavour is due to all these factor (Xiong et al., 2012).
According to medicine value of tea such as demulcent, digestive, diuretic, narcotic, cold, fever, anti-tumors as well as wounds, blood pressure, malaria, ophthalmic, smallpox, sores (Dimitrios., 2006). Tea oil is best raw materials for industrial use and is used to manufacture of soap, margarine, hair oil, lubricants, paint, as well as rustproof oil (Shanan and Ying, 1982). Physicochemical combination of two word physical and chemical study of plant. Nutrition play an important in health, but not providing only vital nutrient. Proximate or physicochemical analysis of edible fruit and vegetable show very critical role in nutritional value. (Pandey et al., 2006). The physicochemical parameter of different tea varieties and clone like moisture content, ash and crude fiber content and fata content. These nutrient are necessary for physiological function of human body.
Objective
The present study developed with the following aims and objectives:
• Physicochemical analysis of various tea varieties and clones.
• To carry out qualitative phytochemical analysis of different tea varieties.
Materials and Methods
Species
Camellia sinensis L. and Camellia assamica L.
Varieties and clones: P3, P5, P7, P8, P9, Qi-Men, Sri-Lankan, Roupi, Chuye, Japanese, Indonesian, Turkish, Jue king.
Sample collection
Tea sample of different varieties and clone were collected form NTHRI, Shinkari, Mansehra. Fresh tea were collected and then washed, dried under sunlight as well as crushed, powder with electrical grinder.
Qualitative phytochemical screening
Phytochemical was chemical compound formed during plant. Plant powder was studied for presence of various phytoconstituents. Extract were made in three solvent that is Aqueous, Methanol and Ethanol. The extracts were used for testing of phytochemicals such as alkaloids, glycosides, Saponins, phenol, flavonoids, tannins, steroid, Terpenoids, phlobatannins, quniones, and anthraquniones as well as carotenoid. Qualitative Phytochemical test were carried out using standard procedure of (Banu and Cathrine, 2015; Balamurugan et al., 2019; Sofoware, 1993).
Chemical and reagents
Some major chemical and reagent were used for phytochemical test such as 50ml ethanol methanol and distilled water. Wagner’s reagent, 10% FeCL3, Concentrated H2SO4, 20% NaOH, 2% HCL, Chloroform, 10% of ammonia, Conc.HNO3, and 85% H2SO4.
Analysis of different constituents
A. Determination of Carotenoids: Extraction of each plant needs 2 ml was used for the determination of carotenoids and by the addition of 5 ml chloroform then treat with 85% of sulphuric acids in it. The presence of blue green colour will be showed carotenoids (Sofoworae, 1993).
B. Test of tannins: In each test tube, 2 ml of aqueous extract was taken and concentrated ferric chloride will be added. Dark green tannins will be indicated the presence of tannins. (Harbone, 1973).
C. Determination of saponins: Saponins presence was determined by the method of (Harbone, 1973). It’s also called forth test because of the forth formation.
D. Determination of phenol: Ferric test was performed for identification of phenol. Few droplets of 10% FeCL3 solution will be added in 2 ml of plant extract. Blue green colour will be indicated the presence of phenol (Sofoworae, 1993).
E. Determination of steroid: Taken 2 ml of ethanolic extract in test tube and added few drop of conc. H2SO4 carefully form the side’s wall of the test tube. The red coloration were showed the presence of steroid (Sofoworae, 1993).
F. Determination of flavonoid: Few drop of concentrated ammonia was added 2 ml of plant extract in test tube and yellow colour indicate the presence of flavonoid (Sofoworae, 1993).
G. Determination of phlobatannins: Few drop of 2% HCL and 1ml of plant extract was added in the test tube. Red precipitate will be showed the presence of phlobatannins (Sofoworae, 1993).
H. Test of alkaloids: 2ml of the plant extract was mixed with 5 ml of (2%) hydrochloric acid in the test tube. boiled for few minute using water bath and filtrated by filter paper. After added 0.5 ml of Wagner’s reagent in the extract. Formation of reddish brown precipitate showed the presence of alkaloid (Idu and Igeleke, 2012).
I. Test of terpeniods: Salkowaki test was performed for identification of terpeniods by Sofoworae, (1993).
J. Determination of glycoside: 5 ml of conc. H2SO4 will be treated with 2 ml plant extract and boiled for 15 minute on Fume mood. After mixture will be cooled and 20% NAOH used and added few drop of FeCL3 in test tube. Green to black colour show the presence of glycoside (Sofoworae, 1993).
K. Determination of quniones: 1ml of concentrated sulphuric acid and 1 ml of plant extract was taken in test tube for detected of quniones. Red precipitate will be showed the presence of quniones (Sofoworae, 1993).
L. Test for anthraquniones: 10% (10 ml) of ammonia solution will be added in 1 ml of each extract of tea sample and pink colour will be showed the presence of anthraquniones. (Sofoworae, 1993).
Physicochemical analysis of tea samples
Leaves of different varieties of tea sample were taken and then powdered as well as used for physicochemical analysis such as moisture content, ash content, fat content and crude fiber. Physicochemical analysis of different tea sample was followed by the standard techniques (AOAC, 2000; Thomas et al., 2008).
A. Chemical reagent: Sulphuric acid, Sodium hydroxide and distilled water.
B. Determination of moisture content: Moisture content of different varieties and clones was followed by standard method of (AOAC, 2000). 3 gram powdered of each tea varieties was weighed in petri dish through electronic balance and dish was transfer in an oven at 105°C for 3 hours. After this, take out the Petri dishes from the oven and cool in Desiccators for 20 minutes and finally reweight.
C. Determination of ash content: Taken 2 gram of each tea sample in the crucible and note the sample weighed. The sample was ready for burning and place the sample containing crucible inside the furnace. After set the temperature at 550°C for 6 hours and then turn “OFF” the power of furnace. Now then, it was transferred from Furnace to Desiccators and cool it (AOAC, 2000).
D. Determination of crude fiber content: Crude fiber content was determined according to the method of (AOAC, 2000). The procedure was determined in two step:
1. Acid Digestion
2. Base Digestion
Acid Digestion (H2SO4)
Take 2 gram of powdered of each tea sample was transferred to beaker, containing 200 ml 1.25% H2SO4 heating on a fume mood at specific temperature 95°C For 2 hours and after then filter by using linen cloth. These residue was washed with hot water to remove acid completely. The residue was transfer to another beaker for alkali digestion.
Base Digestion (NaOH)
The acid digestion residue was transfer to another beaker, containing 200 ml of 1.25% NaOH was again heating on a fume mood and boiled the sample for 2 hours and filtration again, then washed with hot water to remove base residues completely. The residues (crucibles) was placed in an oven at 130°C for 3 hours then transfer into desiccators for cooling and reweight. After this residues was again transfer into muffle furnace at 550°C for one hours then cool in desiccator.
Results
Phytochemical analysis of tea sample
Phytochemicals are the center of phyto-medication. Camellia sinensis L. and Camellia assamica L. as well clone were chosen for phytochemical investigation. Extract were prepared in three solvent that is ethanol, methanol and distilled water. In the current examination a few tea assortments and clone of phytochemical were carried out on each extract for analyzing and identifying different constituent such as Saponins were tried by utilizing froth test, alkaloid were tried by Wagner's test, phenolic compound were test by utilizing phenol test, tried for steroid by utilizing H2SO4 test for tannin by utilizing ferric chloride, flavonoid were test by utilizing acid-alkaline test, terpeniods were test by utilizing Salkowaki test and test for glycoside by FeCl3. The phytochemical screening of ethanolic, methanolic and aqueous extracts of leaf samples of different tea varieties and clone revealed the presence and absence of phytochemicals such as, alkaloids, glycosides, Saponins, phenols, flavonoids, steroid, terpeniods, quniones, anthraquniones, tannins as well as phlobatannins. In methanol, ethanol and aqueous extract of tea sample were showed the presence of phytochemical test include Tannins, Saponins, Flavonoid, steroid, and quniones while flavonoid were indicated absent in Japanese variety in aqueous extract (Table 1). Anthraquniones and alkaloid were found absent in all tea varieties and clone. Phlobatannins were showed negative test in aqueous in all tea sample and positive result were in ethanolic leaves extract as well as few tea sample were absent methanolic extract. The result of qualitative phytochemical test showed in table below:
| Varieties and clones | Plant Extract | Phytochemical test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan | Sap | Phen | Flav | Glyco | Stero | carot | Terp | Phlo | Qun | anthraqu | alkal | |||
| Roupi | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | - | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | + | - | + | - | - | ||
| Qi-men | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | + | - | + | - | - | ||
| Chuye | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | + | + | - | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | - | - | + | - | - | ||
| Jue king | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | + | + | - | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | + | - | + | - | - | ||
| Japanese | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | - | + | - | + | + | + | - | - | ||
| Aq | + | + | + | - | + | + | + | + | + | + | - | - | ||
| Turkish | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | - | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | + | - | - | + | - | - | ||
| Sir lanka | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | + | + | - | + | - | - | ||
| Indonesian | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | - | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | + | + | - | + | - | - | ||
| P-3 | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | + | - | - | + | - | - | ||
| P-5 | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | + | + | - | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | + | - | + | - | - | ||
| P-7 | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | - | + | - | - | ||
| Aq | + | + | + | + | + | + | + | - | - | + | - | - | ||
| P-8 | Mth | + | + | + | + | + | + | + | + | + | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | - | - | + | - | - | ||
| P-9 | Mth | + | + | + | + | + | + | + | + | - | + | - | - | |
| Eth | + | + | + | + | + | + | + | + | + | + | - | - | ||
| Aq | + | + | + | + | + | + | - | + | - | + | - | - | ||
Table 1. Phytochemical Analysis of tea leave varieties and clone using in methanol, ethanol and distilled water result.
Physiochemical analysis of tea sample
The result of physicochemical analysis of different tea verities and clone were showed in table. The moisture content, ash content and crude fiber of tea sample were found in the range of 2.14-2.84%, 0.09%-0.37%, 0.08%-0.34%.
Moisture content
The highest percentage of moisture was showed in Qi-men (2.84%) whereas lowest percentage of moisture content was recorded Sir Lanka (2.14%) tea variety. The result of moisture content below in Fig. 1.
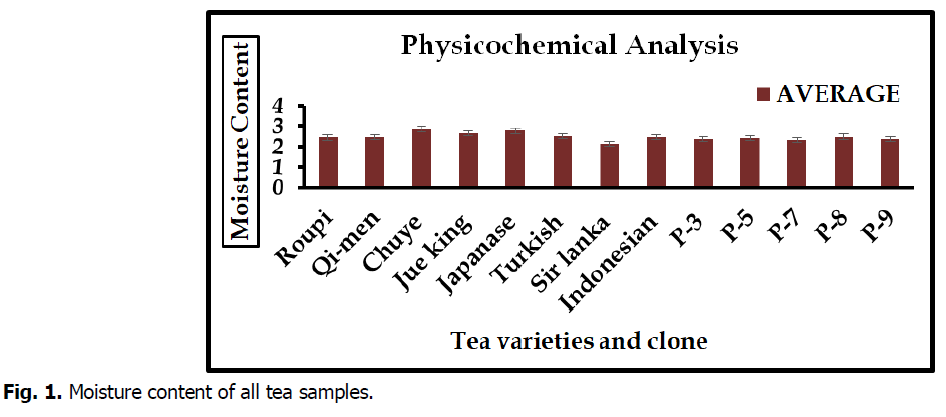
Fig 1. Moisture content of all tea samples.
Ash content
Ash content is also a most second physiochemical parameter. In result of ash content maximum percentage was reported in clone P-8 (0.34%) while minimum in Japanese (0.09%) tea variety. These of all tea sample of ash content were showed in Fig. 2.
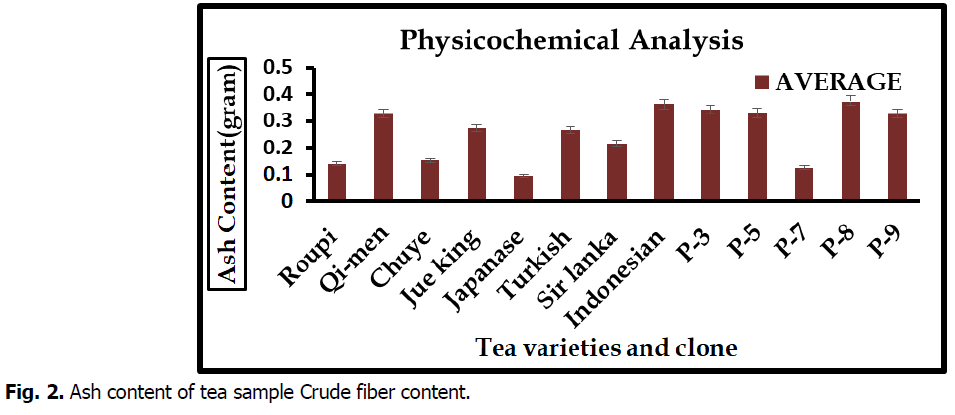
Fig 2. Ash content of tea sample Crude fiber content.
Crude fiber content
Crude fiber content is other important physiochemical parameter the highest percentage in crude fiber content was observed in clone P-5 (0.34%) whereas lowest percentage in Turkish (0.08) tea variety. Different tea varieties and clone result were mention in Fig. 3.
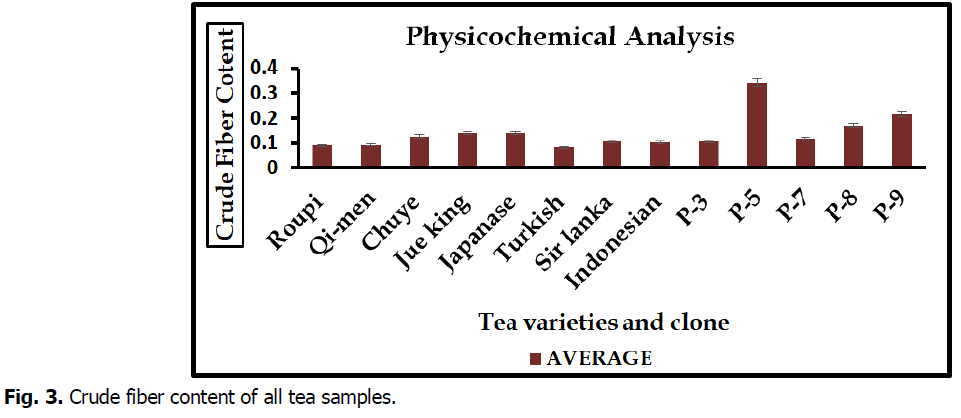
Fig 3. Crude fiber content of all tea samples.
Crude fat content
Crude fat content of tea sample were range from 0.86 to 1.73 respectively. Maximum result of (1.73) crude fat content were observed in Jue king while minimum (0.86) were recorded in Indonesian variety. The outcome was showed in Fig. 4.
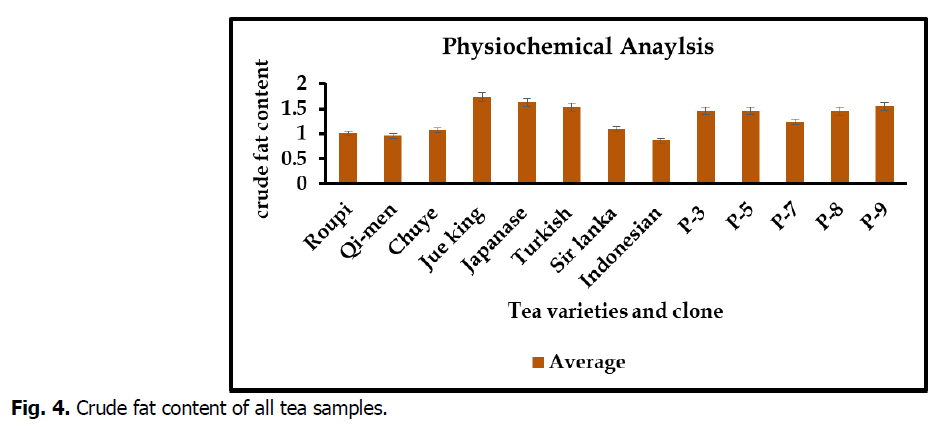
Fig 4. Crude fat content of all tea samples.
Discussion
Phytochemical analysis of tea sample
The present work base on phytochemical test of tea leave of all varieties and clone were done by using the ethanol, methanol and aqueous extract. Numerous studies confirmed the presence of these phytochemicals contribute medicinal as well as physiological properties to the plants studied in the treatment of different ailments (Edeogal et al.,2005). In methanol, ethanolic and aqueous leaves extract of tea samples indicated the presence of phytochemical screening such tannins, Saponins, flavonoid, phenol, steroid, and quniones (Table 1). Similarly present work are agree with those of (Singh et al., 2007) that phenolic compound are considered one main group of secondary metabolites. These result are similar with finding of (Brindha and Arthi, 2010) who concluded that glycoside, flavonoid and Saponins all were present in three extract and glycoside have ability to increase the efficiency of heart beat and impulses. Phlobatannins and alkaloid were found absent in both extract of tea sample. Our outcome are in line with those of (Clinton, C., 2009) who stated that phlobatannins have antioxidant properties. Present study reported that alkaloid were found absent in tea sample in methanol, ethanol, and aqueous leaves extract. Our result showed dissimilarities with work of who (Harborne, 1973) that reported alkaloid have antispasmodic and antibacterial activity.
Physicochemical analysis
The results of physicochemical analysis of plant materials were showed the moisture content, ash content band crude fiber content in figure. Moisture content in different varieties and clone varied from 2.14 to 2.84%. Maximum percentage of moisture content was examined in Qi-men and minimum in sir lanka variety. Present study are similar with pervious of (Kurma et al., 2005) that moisture content of tea totally depend upon nature of tea and drying techniques. Highest (0.37%) ash content was reported in clone P-8 and lowest average (0.09%) in Japanese variety. These result are similar with work of (Dawodu et al., 2013) that reported high ash indicated tea can serve as good basis material. High ash content in tea due to less moisture content in tea and less ash content due to contamination using extract raw material for the product of tea. Crude fiber content minimum percentage (0.08%) was reported in Turkish variety but maximum average (0.34%) were examined in P-5. (Śmiechowska et al., 2006).
Conclusion
The experiments showed the presence of phytochemical constituent like tannins, Saponins, flavonoid, phenol, steroid and quniones in all tea varieties. The presence of these phytochemical involved medicine as well physiological properties to the plant studies in the different cure. Extract form these varieties can be seen as essential source of useful drug, energy and nutrient. The studies also providing good knowledge concerning quality of tea beverage available in Pakistan and great important in pharmaceutical agent.
Acknowledgement
This work was supported by National Tea And High Value Crop Research Institute, Shinkari Mansehra, Pakistan and Department of Botany Hazara University Mansehra, Pakistan for providing the lab material for the activity of the research. I am very thankful to my supervisor Prof. Ghulam Mujtaba and co-supervisor Dr. Abdul Waheed who support every time in lab work.
Funding
Specific funding were not required for this work.
Conflict of Interest
The authors declare no conflict of interest.
References
Akhlas, M. (2003). Qualitative assessment of fresh tea produced in Pakistan growing under different agro-ecological conditions and fertilizer treatments. Pakistan Journal of Botany (Pakistan).
AOAC. (2000). Association official analytical chemist, Gaithersburg, MD, USA.
Banu, K.S., Cathrine, L. (2015). General techniques involved in phytochemical analysis. International Journal of Advanced Research in Chemical Science, 2:25-32.
Brindha, D., Arthi, D. (2010). Antimicrobial activity of white and pink Nelumbo nucifera gaertn flowers. Asian Journal of Pharmaceutical Research and Health Care.
Cabrera, C., Artacho, R., Giménez, R. (2006). Beneficial effects of green tea-a review. Journal of the American College of Nutrition, 25:79-99.
Clinton, C. (2009). Plant tannins: A novel approach to the treatment of ulcerative colitis. Nature Medical Journal, 1:1-4.
Dimitrios, B. (2006). Sources of natural phenolic antioxidants. Trends in Food Science and Technology, 17:505-512.
Dawodu, M.O., Obimakinde, S.O., Olutona, G.O. (2013). Trace metal concentrations in some tea leaves consumed in Ibadan, Nigeria. African Journal of Agricultural Research, 8:5771-5775.
Edeogal, H.O., Okwu, D.E., Mbaebie, B.O. (2005). Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology, 4:685-688.
Harbone, J. (1973). Experimental methods in analytical chemistry. New York, Chapman and Hall, pp:140-145.
Kumar, A., Nair, A.G.C., Reddy, A.V.R., Garg, A.N. (2005). Availability of essential elements in Indian and US tea brands. Food Chemistry, 89:441-448.
Pandey, M., Abidi, A.B., Singh, S., Singh, R.P. (2006). Nutritional evaluation of leafy vegetable paratha. Journal of Human Ecology, 19:155-156.
Sofowora, A. (1993). Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd., Ibadan, pp:191-289.
Singh, R., Singh, S., Kumar, S., Arora, S. (2007). Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food and Chemical Toxicology, 45:1216-1223.
Smiechowska, M., Dmowski, P. (2006). Crude fibre as a parameter in the quality evaluation of tea. Food Chemistry, 94:366-368.
Thomas, S., Patil, D.A., Patil, A.G., Chandra, N. (2008). Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. fruit. Journal of Herbal Medicine and Toxicology, 2:51-54.
Waheed, A., Hina, G., Hamid, F.S. (2017). Molecular characterization of tea clones ‘Camellia sinensis’ grown at NTHRI, Shinkiari. International Journal of Bioscience, 10:142-151.
Wadood, A., Ghufran, M., Jamal, S.B., Naeem, M., Khan, A., Ghaffar, R. (2013). Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochemical and Analytical Biochemistry, 2:1-4.
Xiong, Z., Qi, X., Wei, X., Chen, Z.Y., Tang, H., Chai, S.F. (2012). Nutrient composition in leaves of cultivated and wild Camellia nitidissima. Pakistan Journal of Botany, 44:635-638.
Author Info
D. Kamal1*, G. Mujtabshah1, A. Waheed2, M. Abbass Khan2, N. Ahmed1, U. Jadoon1, M. Iqbal1, A. Lariab Syed1, R. Bibi1, I. Iqra1, A. Qamer1 and I. Ahmed22PARC-National Tea and High Value Crops Research Institute, Shinkiari, Mansehra, Pakistan
Citation: Kamal, D., Mujtabshah, G., Waheed, A., Abbass Khan, M., Ahmed, N., Jadoon, U., Iqbal, M., Lariab Syed, A., Bibi, R., Iqra, I., Qamer, A., Ahmed, I. (2022). Phytochemical and physicochemical variations in different tea varieties and clones grown at Nthri, Shinkiari, Mansehra Pakistan. Ukrainian Journal of Ecology. 12:73-79.
Received: 13-Apr-2022, Manuscript No. UJE-22-60720; , Pre QC No. P-60720; Editor assigned: 15-Apr-2022, Pre QC No. P-60720; Reviewed: 29-Apr-2022, QC No. Q-60720; Revised: 04-May-2022, Manuscript No. R-60720; Published: 09-May-2022, DOI: 10.15421/2022_367
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
