Research - (2021) Volume 0, Issue 0
Physical-chemical water parameters structuring phytoplankton assemblages in endorheic and alkaline lentic system, Lake Rukwa in the Western Rift Valley, Southern Highlands of Tanzania
E. Moto1* and R.S. Maghembe2Abstract
Lake Rukwa is the only endorheic and alkaline lentic system in the Western Rift Valley, in the Southern Highlands of Tanzania. Due to anthropogenic impacts threatening this system, there is a need to understand various factors and processes affecting its phytoplankton community. Phytoplankton were studied in three locations, one offshore (zone 1) and two near river Luika (zone 2) and Songwe (zone 3) in the dry and wet seasons (October 2016 and May 2017), respectively. The study examined the composition, distribution and assemblage structure of phytoplankton in relation to physical water parameters. A total of 19 species belonging to four classes were recorded. These included nine species of Bacillariophyceae (Diatoms), four species of Dinoflagellates, three species of Cyanobacteria and three species of Chlorophytes. The sampling zones differed significantly in phytoplankton composition and richness, with richness being high in offshore open waters than in the river mouths. Spearman’s rank correlation between water parameters and phytoplankton community attributes indicated the influence of physical parameters on phytoplankton distribution.
Keywords
Phytoplankton, Composition, Abundance, Freshwater, Lake Rukwa.
Introduction
Lake Rukwa is the only endorheic and alkaline lentic system in the Western Rift Valley, southern highlands of Tanzania (Haberyan, 1987; WREM, 2013). It is characterized by substantial diversity of species, habitats and by the presence of endemic, rare, and threatened species of fauna and flora (WREM, 2013). Lake Rukwa is one of the most biologically productive ecosystems in the country. It supports various aquatic organisms including freshwater turtles, hippopotamus, crocodiles, fish, various macroinvertebrates and plankton (Ricardo, 1936; Haberyan, 1987). While the lake is still in a relatively pristine state, it is facing many threats including those related to climate change and anthropogenic disturbances from surrounding environments such as mining, livestock keeping and agricultural activities.
Due to inadequate monitoring facilities, limited availability of quality data and accessibility to the lake, little is known about Lake Rukwa. Limited studies has been conducted in Lake Rukwa and most of them relied particularly on water quality. Biological studies focusing on phytoplankton and zooplankton are scanty (reference).
Phytoplankton are essential organisms which contribute to the primary food supply in any aquatic ecosystem. They are the initial biological components from which energy is transferred to higher organisms through food chain (Tiwari and Chauhan, 2006; Saifullah et al., 2014). Physicochemical parameters are the major factors that control the dynamics and structure of the phytoplankton community in any aquatic ecosystem (Hulyal and Kaliwal, 2009). Seasonal variations in these parameters play an important role in the distribution, periodicity, quantitative and qualitative composition of freshwater biota. Thus, knowledge of the physical, chemical and biological characteristics of impacted ecosystems is extremely important (Xavier, 2005).
Several studies on phytoplankton of the Rift Valley soda lakes in East Africa have been conducted in the Eastern Rift Valley with little attention paid to the Western Rift Valley. Those studies include ones at alkaline endorheic Kenyan Rift Valley Lakes such as Bogoria, Nakuru and Elmentaita (Odour and Schagerl, 2008), and alkaline Tanzania lakes including Big Momela, Manyara and Embakai (Kihwele and Moronda, 2004; Lugomela et al., 2006; Kaaya, 2007). However, no study has been conducted on the relationship between physical-chemical water parameters and phytoplankton composition in the Western Rift Valley, particularly Lake Rukwa in Southern highlands of Tanzania. Therefore, the present study was aimed at determining the influence of physical water parameters on phytoplankton composition and distribution in Lake Rukwa, which is vulnerable to anthropogenic pressures. An understanding of the phytoplankton communities over time might help in management and preservation of this fragile habitat.
Materials and Methods
Description of the study site
Lake Rukwa is the fourth largest lake in Tanzania located on the south western regions of Rukwa, Katavi and Songwe at 8000’S 32025’E and 8.000°32.417°E. It is within the Great Rift Valley system. The lake covers an area of about 29,219 square kilometers and lies between Lake Tanganyika and Nyasa (also known as Lake Malawi). The alkaline Lake Rukwa lies at an elevation of about 800 metres (Lake Rukwa Basin Report, 2015). The lake has a large floodplain, and its size varies depending on the amount of rain that falls during rainy season and the water from the inflowing rivers. The Rungwa River flows into the lake from the north, while the Momba River flows from the Western area (WREM, 2013). Three other large inflows come in from the south, namely, the Lupa, Chambua and Songwe Rivers. Although there are many rivers flowing in, Lake Rukwa doesn’t have any outlets. The northern part of the basin is often dry, whilst the western area is shallow. The southern basin is deeper, with an average depth of 4-6 meters, with a maximum depth of 15 meters. A population of about 2.2 million (Census, 2002) relies on Lake Rukwa for various economic activities such as fishing, livestock keeping, agriculture and mining. The lake is surrounded by numerous Typha species and mountainous trees.
Lake Rukwa is situated in a tropical, wet miombo woodland climatic region. The annual rainfall starts in November through May, contributing to increment of water level in the lake. In the southern part of the reservoir the annual average rainfall is about 650 millimeters, while in the northern it is 900 millimeters. The average surface temperature of the lake is between 20 and 35 degrees Celsius. The study area was divided into three zones open waters; Luika River mouth and Songwe River mouth (Fig. 1). Description of the sampling zones is provided in Table 1.
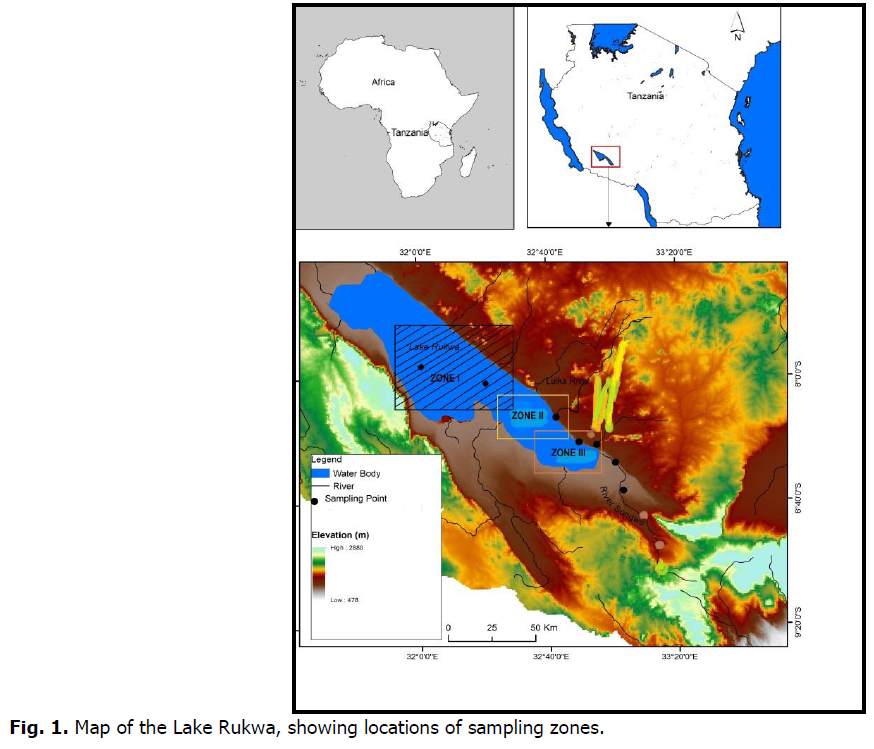
Fig 1. Map of the Lake Rukwa, showing locations of sampling zones.
| Parameter/Season | Dry | Wet | ANOVA F-ratio |
|---|---|---|---|
| pH | 9.22 | 8.50 | 0.29 |
| Temperature (°C) | 28.98 ± 1.90 | 26.40 ± 0.55 | 33.30* |
| Conductivity(μScm-1) | 2200 ± 1.35 | 2000.0 ± 0.65 | 38.90* |
| Turbidity (NTU) | 70.2 ± 0.35 | 120.0 ± 0.55 | 0.75 |
Table 1. Seasonal variations in physical water characteristics of Lake Rukwa in October, 2016 and May, 2017 with one way ANOVA.
Sampling design
Triplicate samples of water quality parameters and phytoplankton were collected in each Zone, covering part of the dry season (October 2016) and part of the wet season (May 2017).
Water samples were collected simultaneously from the same sections that were sampled for phytoplankton and were analysed for physical chemical characteristics. Water quality parameters including pH, conductivity and temperature were recorded in situ. Temperature (°C), and conductivity (μS cm-1) were measured using a hand held refractometer (Model YSI #85/10 FT, USA) while pH was measured using a hand held pH/mV meter (Saxin Model SX 711).
Phytoplankton sampling
The phytoplankton samples were collected during the day between 11.00-14.00 hr by towing a 20 μm mesh size Hydrobios plankton net using a motorized 25 Hp engine boat driven at low speed below the water surface (at a depth of 0.5 m for 5 minutes). Collected samples were immediately transferred to a 250 ml labelled brown glass bottle. Ten samples were collected from each zone, preserved in Lugol’s solution prior to microscopic analysis (Yig˘ it, 2006; Kolo et al., 2010). In the laboratory phytoplankton species were identified by using light microscope at different magnifications (10 X, 40 X and 100 X) and identified using identification keys such as Barber and Haworth (1981); Bellinger, (1992) and Janse Van Vuuren et al. (2006).
Phytoplankton community structure was analysed using three univariate indices (Shannon-Wiener diversity index, Margalef richness index and Pielou’s evenness index) to express the degree of uniformity in the distribution of individuals among taxa in the study area (Imoobe and Adeyinka, 2009).
Statistical analysis
All measured physical parameters, except for pH, were expressed as means ± SE for each sampling station; pH was expressed as median. Phytoplankton abundance data were transformed (using log (x +1)) to meet the statistical criteria for normality. One-way analysis of variance was used for data analysis. Duncan’s multiple range test (DMRT) (Zar, 2001) was done to determine the difference between sampling stations and seasons. Relationships between physical-chemical water quality parameters with diversity indices were determined by a Pearson’s rank correlation test.
Results
Physical parameters
Conductivity and temperature, showed significant variation in both space and time (p <0.05) whereas pH values varied significantly (p <0.05) temporally, but not spatially (Tables 2 and 3, Fig. 2). Conductivity varied significantly between zones (p <0.05). There was a general decline in conductivity over time. The lowest temperature was recorded at Zone II and III and this showed significant difference (p <0.05) from zone I. The highest temperature was recorded in October 2016 and the lowest in May, 2017. The highest pH value was recorded in May 2017 and the lowest in October 2016.
| Taxa | Species | Zone I number/ml |
Zone II number/ml |
Zone III number/ml |
Mean number/ml |
|---|---|---|---|---|---|
| Bacillariophyceae | Coscinodiscus sp Thalassiosira sp Cyclotella sp Stephanodiscus sp Cyclostephanus sp Aulacoseira sp Lauderia sp Skeletonema sp Navicula sp |
1578 (92.22%) 389 (100%) 720 (80.17%) 640 (90.90%) 430 (88.84%) 325 (100%) 400 (100%) 280 (80.92%) 310 (76.16%) |
50 (2.92%) 0.00 90 (10.02%) 64 (9.09%) 54 (11.15%) 0.00 0 34 (9.82%) 44 (10.81%) |
83 (5.85%) 0.00 88 (9.79%) 0.00 0.00 0.00 0 32 (9.24%) 53 (13.02%) |
570 130 299 235 161 108 133 115 136 |
| Dinophyceae | Prorocentrum spp Diplopsalis sp Dinophysis sp Peridinium sp |
1052 (74.71%) 1350 (100%) 980 (91.58%) 826 (86.40%) |
246 (17.47%) 0 90 (8.41%) 0 |
110 (7.81%) 0 0 130 (13.59%) |
469 450 357 318 |
| Cyanophyceae | Anabaena sp Microcystis sp Chroococcus sp |
640 (100%) 648 (100%) 280 (50%) |
0 0 160 (28.57%) |
0 0 120 (21.42%) |
213 216 187 |
| Chlorophyceae | Pediastrum sp Monoraphidium sp Carteria sp |
157 (90.75%) 253 (99.21%) 78 (84.78%) |
16 (9.24%) 0 8 (8.69%) |
0 2 (0.78%) 6 (6.52%) |
58 85 31 |
| Total | 11336 | 856 | 624 | 4272 |
*Values are rated across zones on species basis.
Table 2. Composition, Distribution and relative abundance of phytoplankton species in endorheic Lake Rukwa study Zones (I - III), Tanzania.
| Indices | Zone I | Zone II | Zone III | ANOVA F-ratio |
|---|---|---|---|---|
| Total number of species | 19 | 17 | 16 | - |
| Total number of Individuals | 11336 | 856 | 624 | - |
| Shannon-Wiener Index | 3.201 | 2.872 | 2.890 | 23.56* |
| Pielou Evenness Index | 0.829 | 0.751 | 0.747 | 45.79* |
| Margalef Richness Index | 3.192 | 3.150 | 3.091 | 13.89* |
Table 3. Community attributes of phytoplankton communities in Endorheic Lake Rukwa study Zones (I-III) in October, 2016 and May, 2017.
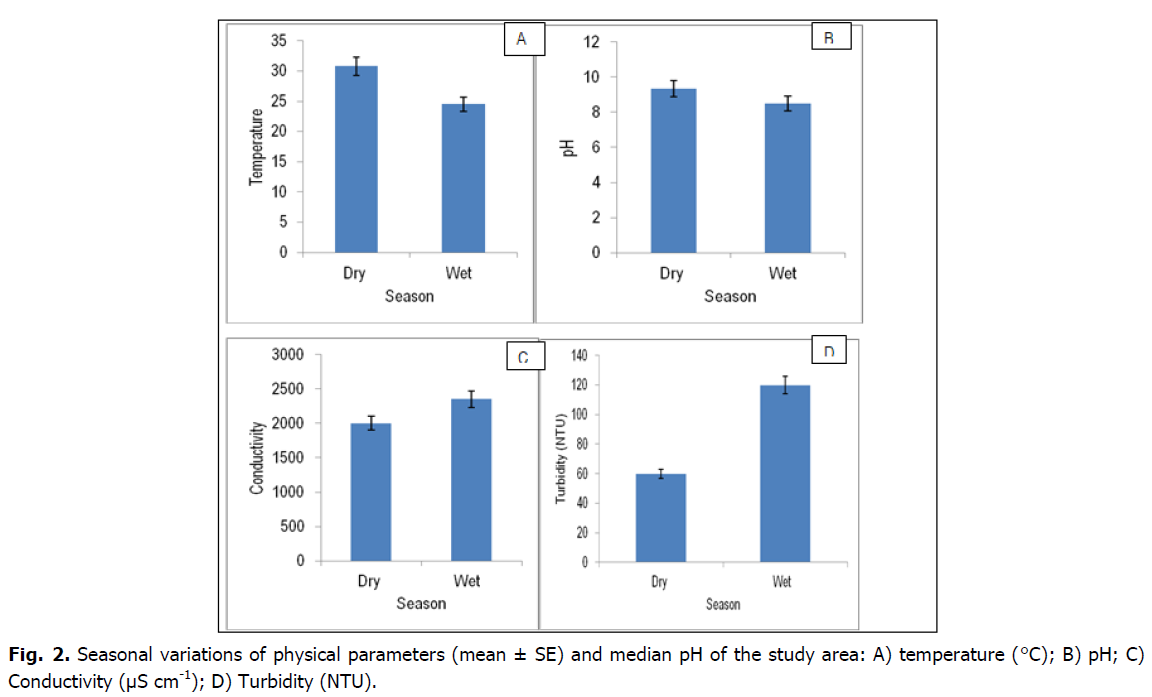
Fig 2. Seasonal variations of physical parameters (mean ± SE) and median pH of the study area: A) temperature (°C); B) pH; C) Conductivity (μS cm-1); D) Turbidity (NTU).
Phytoplankton assemblages
Four classes of phytoplankton comprising of 19 species were identified during the study. These included nine species of diatoms, four species of dinoflagellates, three species of cyanobacteria and three species of chlorophytes. Some species were found in all zones while others were not observed in some zones. Species richness was greatest in Zone I, while few species were found only in Zones II and III.
There were significant differences (p <0.05) among seasons in the diversity indices, as evidenced by the ANOVA. There were differences in abundance between zones and the sampling periods (p <0.05). Zone I had the highest mean abundance per sample (390.7 ± 73.9), whereas zone III had the lowest (98.5 ± 29.4) (Fig. 3). With respect to temporal variation in abundance, October, 2016 had lower mean abundances compared to May, 2017 (Fig. 4).
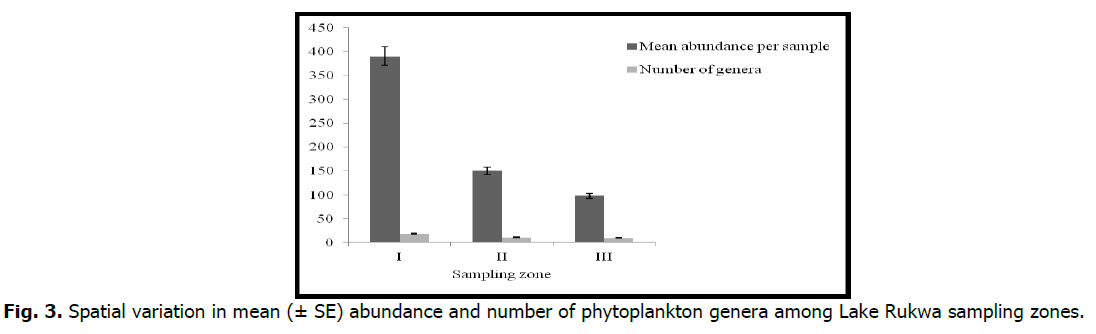
Fig 3. Spatial variation in mean (± SE) abundance and number of phytoplankton genera among Lake Rukwa sampling zones.

Fig 4. Temporal variation in mean (± SE) abundance and number of phytoplankton genera in the endorheic Lake Rukwa.
In all, the diatoms species, Coscinodiscus sp, Cyclotella sp, and Navicula sp were respectively found dominant in Zone I (92.22%), Zone II (10.02%), and Zone III (13.02%), while Navicula sp, Coscinodiscus sp and Coscinodiscus sp had the least relative abundance in Zones I (76.16%), II (2.92%) and III (5.85%), respectively. In the class Bacillariophyceae, the species with the maximum and minimum number of individuals were Coscinodiscus sp (570 individuals/ml) and Aulacoseira sp (108 individuals/ml), respectively. Only one genera of the class Dinophyceae (Prorocentrum spp) was recorded in high numbers in Zone II (17.47%) and had the lowest number in Zone III (7.81%).
In the class Cyanophyceae, three genera (Anabaena, Microcystis, Chroococcus) were recorded in Zone I while only Chroococcus was recorded in Zones II and III. In Zone I Anabaena and Microcystis had the highest relative abundance (100%) each. Chroococcus had the least relative abundance (50%) of genera in this class.
Three genera of the class Chlorophyceae were recorded in this study across the three sampling zones. Carteria, was dominant in Zone III followed by Pediastrum in Zone II and Monoraphidium in Zone I, with each having a relative abundance of 6.52%, 9.24% and 99.21%, respectively.
Phytoplankton species diversity and richness indices
The value of species diversity, richness and evenness indices of phytoplankton in the study area. Shannon-Wienner index, Margalef richness index and Pielou’s evenness index for phytoplankton were 3.201, 3.192 and 0.829 respectively in zone I. 2.872, 3.150 and 0.751 respectively in zone II and 2.890, 3.091 and 0.747 respectively in zone III.
Relationship between physical chemical parameters, phytoplankton abundance and community attributes
Spearman’s rank correlation was performed to assess the relation between physical parameters and phytoplankton community attributes. Abundance correlated positively with most of the physical-chemical water quality parameters except for turbidity and conductivity. Phytoplankton abundance was lowest during the dry period (October). It increased progressively with the onset of the rainy season, but started to decline during peak flows in May. During this period Luika and Songwe Rivers were characterized by large quantities of suspended matter, turbid water and high sediment loads. There was significant negative correlation between turbidity, conductivity and phytoplankton diversity.
Discussion
Four taxonomic groups of phytoplankton were identified in this study and each group was widely distributed in all the sampling zones. However, diatoms dominated the species identified, followed by dinoflagellates, cyanobacteria and chlorophytes. Similar high dominance of diatoms was reported by Oduor and Schagerl, 2008 in the saline, alkaline endorheic Kenyan Rift Valley lakes i.e. Bogoria, Nakuru and Elmentaita. In contrast, it disagrees with the study by Lugomela et al. (2006) in three alkaline lakes of Big Momela, Manyara and Embakai, Tanzania where cyanobacteria dominated the phytoplankton taxa. Phytoplankton variation in Lake Rukwa likely reflects environmental and seasonal differences. The species richness in Rukwa was greater than the 10 species reported by Kihwele and Moronda (2004) from Lake Manyara, but it is less than the 20 species reported by Lugomela et al. (2006) in the Lake Big Momela. Phytoplankton species identified in this study are typical of the Rift Valley soda Lakes in East Africa. According to Oduor and Schagerl (2008) earlier reported the most dominant phytoplankton species in East African freshwater ecosystems are diatoms, cyanobacteria, dinoflagellates and chlorophytes.
According to Jakhar (2013), the phytoplankton community composition in any particular aquatic habitat usually provide information on the prevailing and existing physical and chemical conditions of that habitat. Therefore, the interaction between various physical-chemical water quality parameters can either favour the growth or mortality of phytoplankton, both spatially and seasonally (Yakubu et al., 2000). Phytoplankton have been identified as bio-indicators of aquatic environmental perturbation (Abowei and Sikoki, 2005) because of their easy identification during their period of high density, and high sensitivity to aquatic environmental change compared to other aquatic fauna. Furthermore, the diversity, abundance and seasonality of different phytoplankton groups in aquatic ecosystem affect different biotic components therein, giving them substantial potential in assessing aquatic ecosystem health.
In this study, temporal differences in taxon richness and abundance were recorded between the dry and rainy seasons. Low taxon richness was recorded during the dry period and at the onset of the rains and a significant reduction in abundance was recorded during the peak of rainy season. This finding was in line with those of other studies in saline, alkaline Rift Valley Lakes in East Africa, where phytoplankton abundance was highest at the onset of the rains and declined progressively as the rainfall increased (Kihwele and Moronda 2004; Oduor and Schagerl 2008).
Dry conditions frequently lead to intolerable thermal stress and/or low dissolved oxygen levels for phytoplankton (Nassar, 2000 and Boyce et al., 2010). This could explain the temporal and spatial variation in taxon richness, composition and abundance of phytoplankton in the study area. Sharma et al. (2007) also reported high density of phytoplankton during rainy season in Chandrabhaga River, Garhwal the Himalayas. The environmental health of a particular aquatic ecosystem depends upon spatial–temporal distribution, species composition, relative abundance and biomass of phytoplankton (Khattak et al., 2005). In the present study the phytoplankton abundance showed similar trend between different zones while phytoplankton compositions did not show a trend. This may be due to variations in water quality between different seasons (Pattrick, 1977).
Oduor and Schagerl (2008) reported the prevalence of cyanobacterium species Arthrospira fusiformis in Lake Bogoria as indicator of eutrophic condition in aquatic systems. Furthermore, the occurrence of cyanobacterium Arthrospira fusiformis in Lake Manyara indicated high level organic pollution as a result of high organic matter deposit (Kihwele and Moronda,2004; Lugomela et al. 2006).
The diversity of phytoplankton species in soda lakes is inversely proportional to its abundance (Lugomela et al. 2006; Kaaya, 2007). The high diversity index recorded in Zone I compared to Zones II and III reflected the abundance of phytoplankton in the offshore areas of Lake Rukwa area than in the Luika and Songwe River mouths. High diversity index reported in Lake Manyara of Tanzania was linked to environmental parameters including temperature, light, nutrient availability, salinity, and pH (Lugomela et al, 2006; Kaaya, 2007). However, such high diversity may suggest larger food chain, inter-specific interaction and stability among the soda lake phytoplankton community.
Various studies on phytoplankton of the Rift Valley soda lakes in East Africa such as Nakuru, Bogoria, Elmenteita, Manyara, Natron and Momela reported that the phytoplankton communities in tropical alkaline saline lakes are often enhanced by high nutrient concentrations such as phosphorus and nitrogen, coupled with higher temperature, radiation, abrupt pH variation and salinity (Lugomela et al., 2006; Kaaya, 2007; Oduor and Schagerl, 2008). The dynamics of phytoplankton reflect the function of many environmental factors such as temperature, light, nutrient availability, salinity, pH, and mortality (Chalinda et al., 2004; Kaaya, 2007). In the present study, the composition and abundance of phytoplankton in Lake Rukwa waters were found to vary markedly due to the seasonal environmental fluctuations. It was observed that contribution of each group of phytoplankton in terms of abundance and composition was in the following order: Diatoms>Dinoflagellates>Cyanobacteria>Chlorophytes. High abundance of diatoms and dinoflagellates, in Lake Rukwa waters was due to the predominance period. Some genera occurred during both seasons (dry and wet) including; Thalassiosira, Cymbella, Cyclotella, Stephanodiscus, Cyclostephanus andProrocentrum and were found in all zones although their numbers varied. The observed dominance of these genera is due to their preference of clear water as they are considered omnipresent in the lake waters (Sharma et al., 2007). The increased pH, high temperature and light penetration in Lake Rukwa during dry season, may have also contribute to the high abundance of diatoms and dinoflagellates.
In the current study, Thalassiosira, Cymbella, andProrocentrum, were the most common genera to the other genera. Indeed, Prorocentrum and Thalassiosira are considered to be among the most common genera and are cosmopolitan in the tropics (Jain et al., 2007). Several species such as Thalassiosira, Prorocentrum, Cyclotella, Stephanodiscus, and Cyclostephanos species were found to occur in high numbers contributing substantially to the phytoplankton abundance. Their concentrations reported in this study were relatively higher than those of Microcystis sp, Anabaena sp, and Monoraphidium sp. This may suggest that the tropical waters of Lake Rukwa have high abundance of diatoms species, which may be due to physical-chemical water quality parameters. The low abundance of some phytoplankton in lake waters may also be due to grazing effect by zooplankton and viral infections, which are all well known to influence phytoplankton production in aquatic environment.
Other factors such as concentration of nutrients like nitrate, phosphate, and ammonium also play important roles both in phytoplankton growth and production (Yao et al., 2010). The species of phytoplankton observed in this study have been recorded in other East Africa freshwater lakes like Manyara, Victoria, Nyasa, Tanganyika, and Momela (Lugomela et al., 2006; Jain et al., 2007; Oduor and Schagerl, 2008).
Among the observed species some are potentially harmful blooming microalgae such as Dinophysis, Microcystis, and Anabaena. The competitive advantages of cyanobacteria in the lake may reduce the number of other phytoplankton species due to their ability to regulate their buoyancy and to fix atmospheric nitrogen which in turn add their allelopathic potentials and may further enhance their development even under low nutrient levels (Jacoby et al., 2000; Singh and Balasingh, 2011). Thus when cyanobacteria blooms occur, illumination is reduced in the water column, reducing the growth of other producers that cannot maintain a position near the water surface as they cannot regulate buoyancy (Lugomela et al., 2006).
Conclusion
This study has shown that phytoplankton community of Lake Rukwa is largely dominated by diatoms with Cymbella and Cyclotella being the most prominent genera. Others are cyanobacteria such as Microcystis, Anabaena andChroococcus. These species should be given a special attention due to their ability to cause cyanotoxin pollution. More studies are required on the potentialof harmful phytoplankton which produce cyanotoxin and others which pose threat to other living organisms. The abuandance of phytoplankton in the Lake is a function of physical-chemical water quality parameters. The open water area favoured more diverse species of phytoplankton than other zones. The presence of some phytoplankton in the lake indicated that there was a high level of anthropogenic contamination in and around the water body. This therefore calls for urgent checks on the activities around the lake for sustainable ecosystem health and productivity.
Acknowledgements
We thank the School of Biological Sciences, Department of Biotechnology and Bioinformatics, University of Dodoma, for allowing us to conduct the water quality assessment test and specimen identification at their laboratory. We wish to thank Mr. Eliezer Mwakalapa and Mr. Hatibu Isack, who assisted us in the field and laboratory work, respectively. Great thanks are due to Mr. Makemie Mabula, a GIS specialist of Ilemela District Council for preparing a map of the study area. Last but not least, we thanks the Institute of Marine Sciences (IMS), Zanzibar for providing us the field equipment. This study was self-sponsored and the authors declare that there is no conflict of interest regarding the publication of this paper.
References
Abowei, J.F.N., Sikoki, F.D. (2005). Water Pollution Management and Control. Double trust Publications Company, Nigeria, p:236.
Barber, H.G., Haworth, E.Y. (1981). A guide to the morphology of the diatom frustule. Freshwater Biological Association, Scientific Publication No. 44, Ambleside, UK.
Bellinger, E.G. (1992). A key to Common Algae Freshwater and Some Coastal Species 4th Eds, Institute of water and environmental management, London.
Boyce, D., Lewis, M., Worm, B. (2010). Global Phytoplankton Decline over the past Century. Nature News 466:591-596.
Chalinda, A., Tansakul, R., Angsupanich, S. (2004). Phytoplankton diversity and its relationships to the physico-chemical environment in the banglang reservoir, yala province, songklanakar. Journal of Science and Technology, 26:595-607.
Haberyan, K.A. (1987). Fossil diatoms and paleolimnology of lake rukwa, Tanzania. Freshwater Biology, 17:429-436.
Hulyal, S.B., Kaliwal, B.B. (2009). Dynamics of phytoplankton in relation to physico-chemical factors of almatti reservoir of bijapur district, Karnataka State. Environment Monitor Assessment, 153:45-59.
Imoobe, T.O.T., Adeyinka, M.L. (2009). Zooplankton-based assessment of the trophic state of a tropical forest river. Architech Biological Science, 61:733-740.
Jacoby, J.M., Collier, D.C., Welch, E., Hard, F.J., Crayton, M. (2000). Environmental factors associated with a toxic bloom of Microcystis sp. Canadian Journal of Fisheries and Aquatic Sciences, 57:231-240.
Jain, S.K., Agarwal, P.K., Singh, V.P. (2007). Hydrology and water resources of India. Springer, p:978.
Jakhar, P. (2013). Role of phytoplankton and zooplankton as health indicators of aquatic ecosystem (a review). International Journal of Innovative Research Studies, 2:490-500.
Janse Van Vuuren, S., Taylor, J., Gerber, A., Ginkel, A. (2006). Easy identification of the most common freshwater algae. A Guide for the Identification of Microscopic Algae in South African freshwater.
Kaaya, L.T. (2007). Phytoplankton diversity and productivity with emphasis on the bloom dynamics of the potentially toxic cyanobacteria arthrospira fusiformis, in momela lakes, Arusha Tanzania.
Khattak, T.M., Bhatti, N., Murtaza, G. (2005). Evaluation of algae from the effluent of dandot cement company, dandot, Pakistan. Journal of Applied Science and Environmental Management, 9:147-149.
Kihwele, E.S., Moronda, B.M. (2004). Assessment of water resources, catchment forests river management and conservation in lake manyara basin.
Kolo, R.J., Ojutiku, R.O., Musulmi, D.T. (2010). Plankton communities of Tagwai Dam Minna, Nigeria. Canadian Journal of Fisheries and Aquatic Science, 4:1-7.
Lugomela, C., Pratap, H.B., Mgaya, Y.D. (2006). Cyanobacteria blooms-A Possible Cause of Mass Mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae, 5:534-541.
Nassar, M.Z. (2000). Ecophysiological studies on phytoplankton along the western coast of suez gulf. Philosophy Doctor Thesis, Faculty of Science, Tanta University.
Oduor, S.O., Schagerl, M. (2008). Temporal trends of ion contents and nutrients in three kenyan rift valley saline–alkaline lakes and their influence on phytoplankton biomass. Shallow Lakes, Hydrobiologia, 584:59-68.
Pattrick, R. (1977). Ecology of freshwater diatoms-diatom communities. in: werner, d. (ed.) in: the biology of diatoms. Botanical Monographs, 13:284-332.
Ricardo, C.K. (1936). The fishes of Lake Rukwa. Zoological Journal of the Linnean Society, 40:625-657.
Saifullah, A.S.M., Hena, M.K.A., Idris, M.H., Halimah, A.R., Johan, I. (2014). Diversity of phytoplankton from mangrove estuaries of sarawak, Malaysia. World Applied Sciences Journal, 31:915-924.
Sharma, A., Sharma, R.C., Anthwal, A. (2007). Monitoring phytoplankton diversity in the hill stream chandrabhaga in garhwal the himalayas. Life Sciences Journal, 4:80-84.
Singh, R.P., Balasingh, G.S. (2011). Limnological studies of kodaikanal lake (dindugal district) in special reference to phytoplankton diversity. Indian Journal of Fundamental and Applied Life Sciences, 1:112-118.
Tiwari, A., Chauhan, S.V.S. (2006). Seasonal phytoplanktonic diversity of kitham lake, Agra. Journal of Environmental Biology, 27:35-38.
WREM International. (2013). Lake rukwa basin iwrmd plan: draft interim i report. Water Resources Availability Assessment, p:190.
Xavier, CF. (2005). Avaliação da influência do uso e ocupação do solo sobre a qualidade das águas de dois reservatórios da região metropolitana de Curitiba. Curitiba: Universidade Federal do Paraná–UFPR, p:167.
Yakubu, F., Sikoki, F.D., Abowei, J.F.N., Hart, S.A. (2000). A comparative study of phytoplankton communities of some rivers creeks and borrow pits in the niger delta area. Journal of Applied Science and Environmental Management, 4:41-46.
Yao, P., Yu, Z.G., Deng, C.M, Liu, S.X., Zhen, Y. (2010). Spatial-temporal distribution of phytoplankton pigments in relation to nutrient status in jiaozhou bay, China. Estuarine, Coastal and Shelf Science, 89:234-244.
Yigit, S. (2006). Analysis of the zooplankton community by the shannon-wienner index in kesikko¨pru¨ Dam Lake, Turkey. Tarim Bilimleri Dergisi 12:216-220.
Lake Rukwa Basin Water Board. (2015). Year book-summary of activities for FY 2013/14 for LRBWB. MO Resources in Association with Upscale Innovation-Dar es Salaam.
Author Info
E. Moto1* and R.S. Maghembe22Department of Biological and Marine Sciences, Marian University College, Bagamoyo, Tanzania
Citation: Moto, E., Maghembe, R.S. (2021). Physical-chemical water parameters structuring phytoplankton assemblages in endorheic and alkaline lentic system, lake rukwa in the western rift valley, southern highlands of Tanzania. Ukrainian Journal of Ecology 11 (8), 119-127.
Received: 20-Sep-2021 Accepted: 18-Oct-2021 Published: 25-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
