Research - (2021) Volume 11, Issue 4
Macrobenthic fauna of the coastal sea beds of Oran's Gulf (Western Algeria)
K. Belhadj Tahar1, A. Baaloudj Laboratory of Water, and Environment Biology2, A. Kerfouf Laboratory of Eco-Development of Space1*, M.A. Benallal Laboratory of Eco-Development of Space1 and F. Denis3Abstract
This present work dealing with the study of the benthic community in the Oran's Gulf permits an overview of the macrobenthos encountered in this area, which seems to be more or less threatened by industrial and urban pollution. This study aimed to establish an initial reference state of coastal environments to contribute to the knowledge necessary for their sustainable management. The inventory of the macrobenthic fauna of the Gulf of Oran carried out in 2019 identified 618 macrobenthic individuals, divided into four taxa (531 polychaetes, 38 crustaceans, 31 mollusks, and 18 echinoderms), distributed in 8 sites. The taxonomic diversity included 60, 23, 15, and 9 species of polychaetes, crustaceans, mollusks, and echinoderms, respectively. All this information obtained made it possible to estimate the diversity of macrobenthic invertebrates and characterize the main communities in the coastal sea bed of the Gulf's Oran.
Keywords
macrobenthos, invertebrates, polychaetes, mollusks, crustaceans, echinoderms, Oran's Gulf, west coast Algeria.
Introduction
Most of the previous inventories of the benthic fauna of continental shelf in the west coast of Algeria have not been updated (Pallary, 1900; 1935; Llabador, 1935; Maurin, 1962; Vaissiere and Fredj, 1963; Amar, 1998; Grimes, 2010; Boukhari Benamara, 2014; Meziane et al., 2018; 2020). The prospecting of about eight stations of the continental shelf of the Gulf of Oran allowed the study of the macrobenthic communities, established the faunistic inventory of the species, and specified the biodiversity of the various seabeds of the Gulf. Macroinvertebrates are fundamental elements in the functioning of aquatic ecosystems and keep an essential role in biological balance and trophic chains. The analysis and use of the inventory of identified species carried out make it possible to define the geographical zoning and priority habitat types for the conservation of the biodiversity of macroinvertebrates in these coastal areas and to understand the place of macroinvertebrates in the functioning of biological resources, to identify its ecological values and disturbances caused by human activities (Rebzani-Zahaf et al., 1997; Donga, 2021).
Materials and Methods
Study area
On the Algerian Mediterranean coast, the Gulf of Oran is located between the Gulf of Arzew to the east and Cape Falcon to the west (Leclaire, 1972). The study area is located in the Gulf region (Annex Table 1) and includes eight coastal stations (Figure 1). The Gulf of Oran is supplied by waters that originated from the Atlantic Ocean. The circulation seems to be very turbulent along the African continent. These turbulences favor the dispersion of eventual pollution sources and permit a relatively significant alimentary chain development (Millot et al., 1989). The Gulf of Oran is exposed to pollution and other degradation resulting from the development of multiple socio-economic activities: urbanization, tourism, fishing, and commercial ports (Djad et al., 2015; Tayeb et al., 2015; Mehtougui et al., 2018; Kies et al., 2020).
Figure 1: Sampling area.
Sampling
For each station, 0.2 m2 area was sampled. The individuals collected are stored in formalin and identified in the laboratory using various documents and determination keys. According to their belonging to one of the greatly following zoological groups, a first selection permits species segregation: Molluscs, Crustaceans, Echinoderms, and Annelids Polychaetes. Each individual of every zoological group is identified according to several determination keys, such as Fauvel (1923 and 1927), Picard (1965), Fauchald (1977), Glémarec (1969 and 2000), Kerfouf et al., (2007) Each inventoried species is noted to make species abundances and dominance calculations (Pielou, 1966) and the diversity index (Shannon et al., 1963).
Only one type of sampling device was used: the Aberdeen or Smith-McIntyre grab, for the operations sediment and benthos sampling.
Results and Discussion
The particle size analysis allowed the determination of the sedimentary facies of the stations. The fine sand constitutes the substrate of the majority of the stations, except stations 7 and 8, made up of gravel. Station 5, near the central wastewater discharge of the city of Oran, contains reduced black muddy sediment. The collection of faunistic data allowed the inventory of 618 macrobenthic individuals (Annex Table 2). The inventoried macrofauna for our study area is divided into four taxa (polychaetes, mollusks, crustaceans, and echinoderms). Concerning the zoological composition of the macrobenthic fauna, we noted the significant domination of Polychaetes (531 specimens) in all macrobenthic communities of the Gulf of Oran, followed by crustaceans (38 specimens, Figure 2).
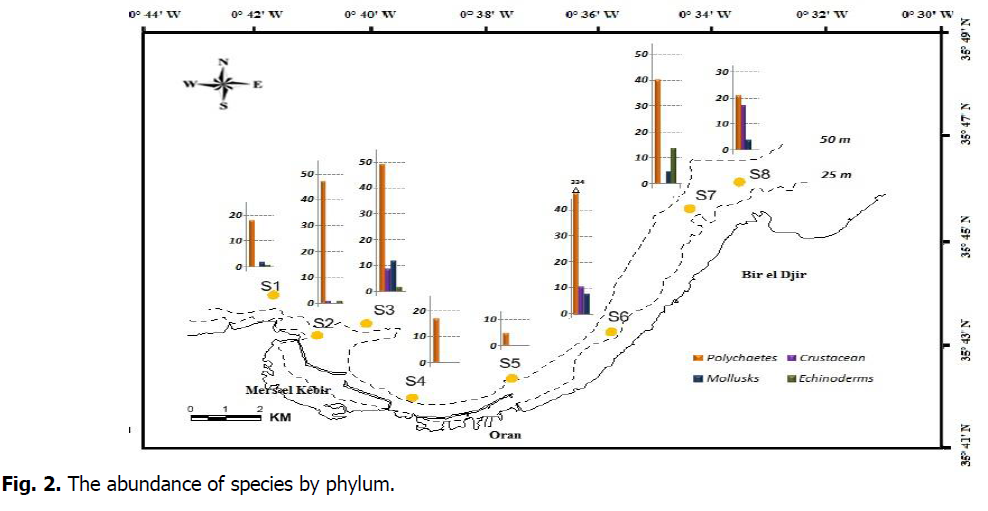
Figure 2: The abundance of species by phylum.
Concerning the specific richness, polychaetes dominate, except for stations S7 and S8, where crustaceans are most represented (Figure 3).
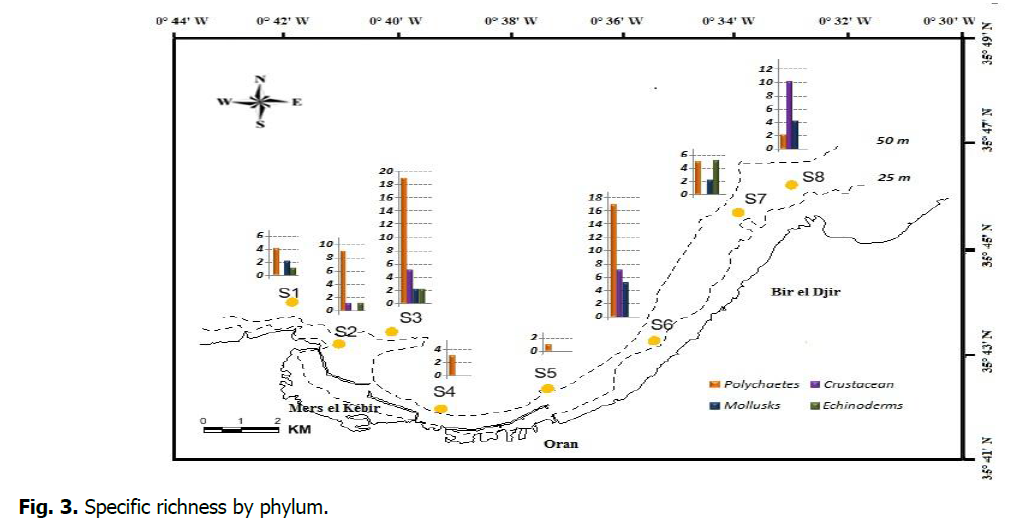
Figure 3: Specific richness by phylum.
Qualitatively, macrobenthic communities are characterized by poor distribution of individuals among the inventoried species. The S6 station, near the wastewater discharge, is the most diverse. Quantitatively, the fauna of stations S4 (3 species) and S5 (one species, H' and J’=0) near Oran's harbor is the least important (Table 1). Only one species at station S5: Pista sp. (Figure 4).
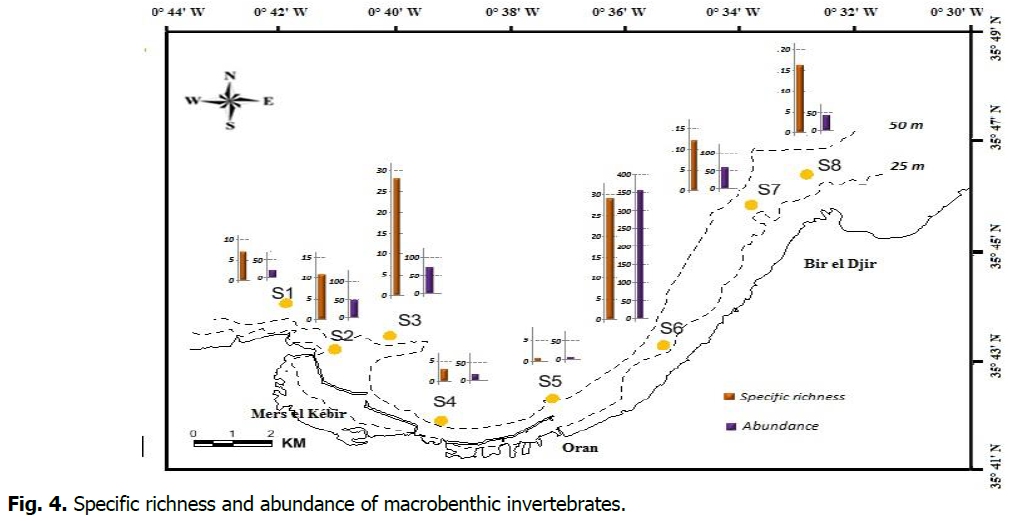
Figure 4: Specific richness and abundance of macrobenthic invertebrates.
| Stations | Specific richness S |
Shannon index H' |
Pielou's eveness J' |
|---|---|---|---|
| S1 | 7 | 2.20 | 0.49 |
| S2 | 11 | 1.97 | 0.43 |
| S3 | 28 | 3.92 | 0.87 |
| S4 | 3 | 1.09 | 0.24 |
| S5 | 1 | 0 | 0 |
| S6 | 29 | 2.18 | 0.48 |
| S7 | 12 | 2.99 | 0.66 |
| S8 | 16 | 2.91 | 0.64 |
Table 1. Site-specific diversity.
Concerning the zoological composition of the population, polychaetesthe manifest dominance of the Polychaetes in the whole of the macrobenthic communities of the Gulf of Oran has been noted. Qualitatively, the Polychaetes are also the most diversified zoological group in all populations, followed by the crustaceans one.
According to their abundance, the classification of species is linked to environmental conditions, in particular abiotic factors: nature of the substrate, hydrodynamics, pollution (Glémarec, 2000). In the Gulf of Oran, the Polychaetes dominate the population on the sandy bottoms, both qualitatively and quantitatively. Ecologically, species with a broad ecological distribution as Hyalinocea bilineata and tolerant sand as Pista cristata are dominant. Comparatively, on the other Algerian west coast studied (Mostaganem, Arzew, Oran), the area of Oran is the poorest both qualitatively and quantitatively (Amar, 1998; ANAT 2004; Grimes, 2010; Kies et al., 2020).
Conclusion
Bionomic research is rarely studied and fragmented. This study on the benthic macrofauna sampled on the Gulf of Oran constitutes an inventory of the coastal ecosystem and complements the database because bionomic research is rarely studied and fragmented. This inventory of ecological interest constitutes a database with lists of fauna and distribution maps of the macrofauna of Oran's Gulf. This study also provides a clear description of the main characteristics of macrozoobenthos communities.
References
Amar Y. (1998) Etude des peuplements macrobenthiques du golfe d’Arzew. Thèse de Magistère, ISMAL (Alger).
ANAT, (2004).Etude relative à la protection et la valorisation du littoral de la wilaya d’Oran. Ministère de l’Aménagement du Territoire et de l’Environnement, 139p.
Boukhari, M. (2014) Les macro-invertébrés du littoral Ouest Algérien: état des lieux et des connaissances. Mémoire de magistère, université de Sidi Bel Abbès, 174p.
Djad Mohamed El Amine, Hassani Maya Meriem, Kerfouf, A. (2015). Bacteriological Quality of Seawater Bathing Areas along the Oran Coast (West northern Algeria). International Journal of Sciences: Basic and Applied Research (IJSBAR), 23(1), 268-275. Donga, Jian-Yu., Zhaod, L., Suna, X., Hub, Ch., Wangb, Y. et al. (2021). Response of macrobenthic communities to heavy metal pollution in Laoshan Bay, China: A trait-based method. Marine pollution bulletin, 167.
Grimes, S. (2010) Peuplements benthiques des substrats meubles de la cote Algerienne: Taxonomie, structure et statut écologique. Thèse de doctorat en sciences, université d’Oran.
Kerfouf, A., Amar, Y., Boutiba, Z. (2007). Distribution of macrobenthos in the coastal waters in the gulf of Oran (Western Algeria) ). PJBS: Pakistan Journal of Biological Sciences, 10 (6): 899-904.
Kies F., Kerfouf A., Elegbede I., Matemilola S., De Los Rios Escalante P., Khorchani, A., Savari, S. 2020. Assessment of the coastal and estuarine environment quality of western Algeria using the bioindicator Polychaeta; the genus Nereis. J. Mater. Environ. Sci., 11(9), 1472-1481.
Leclaire, L. (1972). Holocene sedimentation on the southern most slope of the basin Algéro-Balearic Islands (Algerian precontinent). Memory of the National Natural history museum of Natural History, series. C, Tome XXIV, Paris, 372-3
Llabador F. 1941. Les Echinodermes de l’Oranie occidentale. Bulletin de la société de Géographie et d’Archéologie de la Province d’Oran. Oran.
Maurin, C. 1962. Etude des fonds chalutables de la méditerranée occidentale (écologie et pêche). Résultats des campagnes des navires océanographiques président-Théodore- Tissier 1957 à 1960 et Thalassa 1960 et 1961. Rev. Trav. Inst. Pêches marit. , 26, (2), 163-218.
Meziane, K., Kerfouf, A. 2018 - Current Situation of Stramonita heamastoma (Linnaeus 1758) (Gasteropod Mollusk) in the Western Coast of Algeria. International Journal of Sciences Basic and Applied Research (IJSBAR), 39(2), 40-46.
Meziane K., Kerfouf A., Baaloudj A. 2020. Checklist of gastropod molluscs in west coast of Algeria. Int. J. Aquat. Biol, 8(3), 224-227. Millot, J. C., Taupier-Letage, L., Benzohra, M. (1989). The Algerian Eddles. Earth Sciences Reviews, 27, 17.
Pallary, P., 1900. Coquilles marines du littoral du Département d'Oran. Journal de Conchyliologie, 48(3), 211-422. Pallary, P. 1935. Echinodermes du golfe d’Oran. Bull. Soc. Hist. nat. Afr. N., 35bis, 77
C., Bellan G ., Romano J.-C., (1997). Macrobenthic communities in Algiers harbour. Oceanologica Acta 20(2):461-477 Shannon. F.P, Weaver. W. (1963). The mathematical theory of communication. University of Illinois Press. Urbana: 117p
Tayeb, A., Chellali, M.R, Hamou, A., Debbah, S. (2015). Impact of urban and industrial ef?uents on the coastal marine environment in Oran, Algeria. Mar. Pollut. Bull.
Vaissière R., Fredj, G. 1963. Contribution to the study of the benthic fauna of the continental shelf of Algeria. Bull. Oceanog. Inst. Monaco, 60, 83-83.
| Stations | Latitude N | Longitude W | Depht (m) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 35°44’45’’ | 00°42’15’’ | 70 | |||||||||||||
| S2 | 35°44’03’’ | 00°41’18’’ | 46 | |||||||||||||
| S3 | 35°44’18’’ | 00°40’45’’ | 61 | |||||||||||||
| S4 | 35°42’30’’ | 00°39’33’’ | 42 | |||||||||||||
| S5 | 35°43’05’’ | 00°37’45’’ | 56 | |||||||||||||
| S6 | 35°44’08’’ | 00°35’47’’ | 39 | |||||||||||||
| S7 | 35°46’25’’ | 00°34’29’’ | 60 | |||||||||||||
| S8 | 35°47’10’’ | 00°33’20’’ | 32 | |||||||||||||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |||||||||
| A | D | A | D | A | D | A | D | A | D | A | D | A | D | A | D | |
| Polychètes | ||||||||||||||||
| Amphicteis gunneri (M. Sars, 1835) | 1 | 1.39 | ||||||||||||||
| Amphitrite gracilis (Grube, 1860) | 1 | 1.39 | ||||||||||||||
| Chloeia venusta (Quatrefages, 1866) | 1 | 4.8 | 2 | 4 | 1 | 5.9 | ||||||||||
| Chone duneri (Malmgren, 1867) | 1 | 1.39 | 43 | 12 | ||||||||||||
| Clymene oerstedii (Claparède, 1863) | 2 | 2.78 | ||||||||||||||
| Clymene lumbricoides (Quatrefages, 1866) | 1 | 0 | ||||||||||||||
| Clymene santanderensis (Rioja, 1917) | 3 | 14 | 1 | 0 | ||||||||||||
| Clymene sp | 2 | 1 | ||||||||||||||
| Eunice vittata (Delle Chiaje, 1828) | 10 | 48 | 1 | 2 | 6 | 8.33 | 4 | 24 | 16 | 5 | 17 | 29 | ||||
| Glycera convoluta (Keferstein, 1862) | 2 | 2.78 | 5 | 1 | ||||||||||||
| Glycera lapidum (Quatrefages, 1866) | 2 | 2.78 | ||||||||||||||
| Glycera sp | 6 | 2 | ||||||||||||||
| Goniada norvegica (Ã?rsted, 1845) | 1 | 1.39 | ||||||||||||||
| Harmothoe sp | 1 | 0 | ||||||||||||||
| Harmothoe spinifera (Ehlers, 1864) | 2 | 1 | ||||||||||||||
| Hyalinoecia bilineata (Baird, 1870) | 4 | 19 | 32 | 65 | 20 | 27.8 | 12 | 71 | 229 | 65 | 12 | 20 | 20 | 48 | ||
| Hyalinoecia fauveli (Rioja, 1918) | 5 | 10 | 3 | 4.17 | ||||||||||||
| Hyalinoecia sp | 1 | 2 | ||||||||||||||
| Lanice conchilega (Pallas, 1766) | 1 | 1.39 | ||||||||||||||
| Lepidasthenia maculata (Potts, 1910) | ||||||||||||||||
| Lumbrineris latreilli (Audouin & Milne Edwards, 1833) | 3 | 6 | ||||||||||||||
| Lumbrineris fragilis (O.F. Müller, 1776) | 2 | 2.78 | 17 | 5 | ||||||||||||
| Lumbrineris funchalensis (Kinberg, 1865) | 1 | 0 | ||||||||||||||
| Lysidice ninetta (Audouin & H Milne Edwards, 1833) | 1 | 0 | ||||||||||||||
| Melinna palmata (Grube, 1870) | 1 | 1.39 | ||||||||||||||
| Nematonereis unicornis (Schmarda, 1861) | 1 | 2 | 2 | 1 | ||||||||||||
| Nereis caudata (sensu Delle Chiaje, 1827) | 1 | 2 | 1 | 1.39 | 4 | 6.8 | ||||||||||
| Notomastus lineatus (Claparède, 1869) | 1 | 1.39 | ||||||||||||||
| Petta pusilla (Malmgren, 1866) | 1 | 1.39 | ||||||||||||||
| Pista cristata (Müller, 1776) | 1 | 1.39 | 4 | 1 | ||||||||||||
| Pista sp | 5 | # |
||||||||||||||
| Polynoe scolopendrina (Savigny, 1822) | 1 | 1.7 | ||||||||||||||
| Sabella pavonina (Savigny, 1822) | 2 | 1 | ||||||||||||||
| Sigalion squamatum (Delle Chiaje, 1830) | 1 | 3 | ||||||||||||||
| Sthenelais boa (Johnston, 1833) | 1 | 2 | ||||||||||||||
| Stylarioides plumosa (O.F. Müller, 1776) | 1 | 0 | ||||||||||||||
| Syllis (Typosyllis) amica (Quatrefages, 1866) | 1 | 1.39 | 6 | 10 | ||||||||||||
| Trichobranchus glacialis (Malmgren, 1866) | 1 | 1.39 | ||||||||||||||
| Crustacées | ||||||||||||||||
| Ampelisca multispinosa(Santini&Malka, 1977) | 2 | 5 | ||||||||||||||
| Ampelisca sp | 5.9 | |||||||||||||||
| Ampelisca spinipes (Boeck, 1861) | 1 | 2 | ||||||||||||||
| Anonyx sarsi (Steele & Brunel, 1968) | 1 | 2 | ||||||||||||||
| Apseudes holthuisi Bacescu, 1961) | 24 | 5 | 1 | |||||||||||||
| Atylus falcatus Metzger, 1871) | 1 | 0 | ||||||||||||||
| Bathyporeia guilliamsoniana (Spence Bate, 1857) | 2 | 5 | ||||||||||||||
| Cirolana borealis (Lilljeborg, 1851) | 3 | 4.17 | 1 | 0 | ||||||||||||
| Corophium rotundirostre (Stephensen, 1915) | 71 | |||||||||||||||
| Cypridina mediterranea (Costa, 1845) | 2 | 5 | ||||||||||||||
| Ebalia edwardsi(O.G. Costa, 1838) | 1 | 0 | ||||||||||||||
| Eurydice pulchra (Leach, 1815) | 1 | 0 | ||||||||||||||
| Eurydice sp | 3 | 4.17 | 1 | 0 | ||||||||||||
| Galathea intermedia (Lilljeborg, 1851) | 1 | 1.39 | ||||||||||||||
| Gastrosaccus sanctus (Van Beneden, 1861) | 1 | 2 | ||||||||||||||
| Haplostylus normani (G.O. Sars, 1877) | 1 | 2 | ||||||||||||||
| Harpinia crenulata (Boeck, 1871) | 1 | 0 | ||||||||||||||
| Lophogaster typicus (M. Sars, 1857) | 1 | 1.39 | ||||||||||||||
| Maera shieckei (Karaman & Ruffo, 1971) | 1 | 2 | ||||||||||||||
| Nebalia bipes (Fabricius, 1780) | 1 | 1.39 | ||||||||||||||
| Paguristes oculatus (Fabricius, 1775) | 1 | 2 | ||||||||||||||
| Urothoe brevicornis (Spence Bate, 1862) | 5 | 12 | ||||||||||||||
| Urothoe elegans (Spence Bate, 1857) | 1 | 2 | ||||||||||||||
| Molluscs | ||||||||||||||||
| Corbula gibba (Olivi, 1792) | 1 | 2 | ||||||||||||||
| Gari costulata (W. Turton, 1822) | 1 | 2 | ||||||||||||||
| Hinia reticulata (Linnaeus, 1758) | 1 | 0 | ||||||||||||||
| Leda fragilis (Chemnitz, 1784) | 1 | 4.8 | 11 | 15.3 | ||||||||||||
| Limatula subauriculata (Montagu, 1808) | 1 | 1.39 | 1 | 1.7 | 1 | 2 | ||||||||||
| Loripes lacteus (Linnaeus, 1758) | 1 | 0 | ||||||||||||||
| Lyonsia norvegica (Chemnitz, 1788) | 1 | 4.8 | ||||||||||||||
| Nucula sulcata (Bronn, 1831) | 4 | 6.8 | ||||||||||||||
| Nucula turgida(Gould, 1846) | 3 | 1 | ||||||||||||||
| Peplum clavatum (Poli, 1795) | 1 | 0 | ||||||||||||||
| Callista chione pitaria (Linnaeus, 1758) | 1 | 2 | ||||||||||||||
| Venericardia antiquata (Linnaeus, 1758) | 2 | 1 | ||||||||||||||
| Echinodermes | ||||||||||||||||
| Amphiura chiajei (Forbes, 1843) | 1 | 4.8 | 2 | 3.4 | ||||||||||||
| Amphiura lacazei (Guille, 1976) | 1 | 1.39 | ||||||||||||||
| Amphiura mediterranea (Lyman, 1882) | 3 | 5.7 | ||||||||||||||
| Astropecten irregularis (Pennant, 1777) | 1 | 2 | ||||||||||||||
| Echinocyamus pusillus (O.F. Müller, 1776) | 1 | 1.39 | ||||||||||||||
| Ophiopsila aranea (Forbes, 1843) | 1 | 1.7 | ||||||||||||||
| Ophiura albida (Forbes, 1839) | 1 | 1.7 | ||||||||||||||
| Psammechinus microtuberculatus (Blainville, 1825) | 7 | 12 | ||||||||||||||
Annex Table 1: Geographic and bathymetric coordinates of sampling stations.
Author Info
K. Belhadj Tahar1, A. Baaloudj Laboratory of Water, and Environment Biology2, A. Kerfouf Laboratory of Eco-Development of Space1*, M.A. Benallal Laboratory of Eco-Development of Space1 and F. Denis32University 8 may 1945, Guelma, 24000, Algeria
3Department of Aquatic Environments and Populations, UMR 7208 'BOREA', MNHN, 29182, Concarneau, France
Citation: Belhadj Tahar, K., Baaloudj, A., Kerfouf, A., Benallal, A.M., Denis, F. (2021). Macrobenthic fauna of the coastal sea beds of Oran's Gulf (Western Algeria). Ukrainian Journal of Ecology, 11 (4), 1-4.
Received: 20-Apr-2021 Accepted: 08-Jun-2021 Published: 30-Jun-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
