Research - (2021) Volume 0, Issue 0
Influence of mineral fertilizers and inoculants on the yield and quality of chickpea grain
S. Burykina1*, A. Kryvenko1*, I. Gulyaeva2, V. Gamayunova3 and A. Shepel4Abstract
We studied the combined effect of mineral fertilizers and pre-sowing bacterization of chickpea seeds on the processes of nodule formation, grain productivity, and seed quality during 2016-2018. Natural and climatic zone is the Steppe; agro-soil province is the dry Black Sea Steppe. Soil-southern chernozem low humus heavy loamy low provided with mineral nitrogen and medium provided with available forms of phosphorus and potassium. As a result of the research, it was established: according to the totality of characteristics, the conclusion is distinguished: application of P30K30 for pre-seed cultivation+N30 in the branching phase in combination with seed bacterization; the increase in yield was 1.50 t/ha or 16.7 kg per unit of active fertilizer substance compared to the variant without fertilizers;-on average, according to seed bacterization, compared to P30K30, a single top dressing of chickpea plants in the branching phase with mineral nitrogen at a dose of 30 kg/ha provided a payback of a unit of active nitrogen substance-8.3 kg;-in fields with an average and higher content of available forms of phosphorus and potassium, double application is effective: N30 in the branching phase+N30 at the beginning of the bluff. Pre-sow inoculation of chickpea seeds with BTU-inoculant and Rhizobofit® preparations is combined with them. The increase in yield is 0.99-0.89 t/ha, the return of a unit of fertilizers with chickpea grain is 16.5-14.8 kg, and on average for bacterization, the return is 5.8 kg;-increasing the dose of the pre-sowing application from N30 to N120 suppresses the process of nodule formation without seed bacterization by 32.3%, using inoculants-from 15.8% to 54.3%, and their mass by 53.6% and from 21.4% to 60.0%, respectively.
Keywords
Chickpeas, Fertilizers, Inoculation, Yield, Protein, Weight of 1000 seeds.
Introduction
Awakening of interest in the long-known, but until recently uncommon culture, caused by actual and projected climate change in the southern region of Ukraine and its unique nutritional qualities. We are already seeing sharp changes in the weather. Thus, in the south of Ukraine, in the particular in Odesa, Mykolaiv and Kherson regions, there are quite severe frosts in winter with sudden, rather prolonged thaws, the number of abnormally hot days in summer increases, and the strength and frequency of droughts increase (Kulbida et al., 2013). With the increase in the total amount of precipitation during the agricultural year, its frequency decreased and the intensity of rains increased (in some months up to 80% of precipitation fell in the form of showers, the rest unproductive), the distribution of precipitation on the vegetation of major field crops worsened; compared with the period of 1961-1990, in 2017 there was a significant deterioration of HTC (Stepanenko et al., 2014; Burykina and Tsurkan, 2020). According to scientists, these phenomena will deepen by 2050, most of these areas are deserted (Polevoy and Bozhko, 2015). Producers will be forced to change the set of crops, technology, and varietal policy.
In view of the above, chickpeas are a promising crop because they can withstand air and soil drought due to a well-developed root system and economical water consumption per unit area (Sichkar and Bushulyan, 2007). In the south for Ukraine, the technology of growing chickpeas is insufficiently studied. Regarding the fertilizer system, it should be noted that there is no consensus, both on the feasibility of their use under chickpeas and in relation to the rules and timing of application. It is necessary to study this aspect of chickpea cultivation technology in the rainfall conditions of the southern steppe taking into account the predicted global climate changes and the results of research by personal and other authors (Lavrenko,2015; Tomnitsky, 2012).
It is believed that there are no aboriginal nodule bacteria in the soils of Ukraine, only in some places where this culture was grown there are local populations of Mesorhizobium ciceri. For the formation of nitrogen-fixing legume-rhizobial system, providing plants with molecular nitrogen in the air requires nitragination-pre-sowing seed treatment with biological products, which are based on selected strains of chickpea nodule bacteria (Didovich et al., 2012; Shcherbakova, 2015). The introduction of mineral nitrogen has an ambiguous effect on the mechanism of nitrogen fixation (Tolkachev et al., 2003). It is necessary to find ways to rationalize the combination of symbiotic and autotrophic nutrition of legumes. In this case, it will be done on chickpea crops. The aim of the work is to determine the optimal combination of the chickpea fertilizer system with inoculant for the arid conditions of the southern steppe.
Materials and Methods
Studies on the influence of mineral fertilizers on the formation of chickpea productivity and seed quality were conducted during 2016-2018 by setting up a two-factor field experiment in the experimental field of the Odessa State Agricultural Experimental Station of the National Academy of Sciences. The natural and climatic zone is the steppe, the agro-soil province is SS-1 (dry steppe of the Black Sea). The indicator of the new vegetation period is insanely atmospheric. However, the effectuality of the fall in a significant world is to lie in the middle of winter, which means in the middle of the temperature regime, which is to be stored by the stretch of vegetation.
To characterize drought, the following indicators of hydrothermal coefficients are determined: HTC less than 0.5-strong dryness; HTC 0.6-0.7-very dry; 0.8-0.9-dry; 1.0-1.2-lack of moisture; 1.3-1.6-middle moisture; >1.7-high moisture (Kulbida, Elistratova and Barabash, 2013). For the vegetation period of these indicator chickpeas, determined in the middle as lack of moisture (2016), dry (2017) and strong dry-2018 (Fig. 1). The soil of the experimental site is southern low humus-heavy Loess chernozem, low-supplied with nitrate nitrogen and medium-supplied with available forms of phosphorus and potassium. Repetition in the experiment is done 4 times; the arrangement of variants was carried out by the method of split sections in accordance with recognized methods of setting up field experiments (Dospekhov, 1985). The accounting area of first-order land plots is 80 m2; second-20.6 m2.
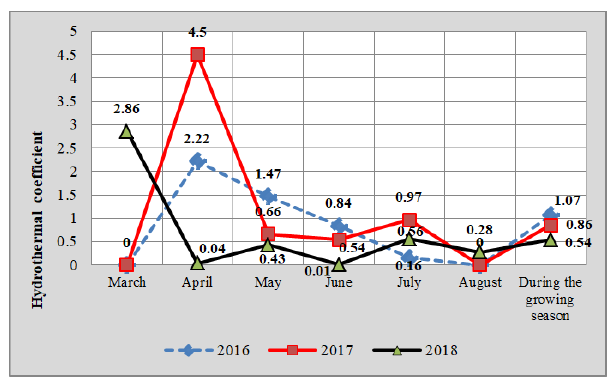
Fig 1: Hydrothermal coefficient.
In the field experiment, the variants of mineral fertilizers (factor a) presented in Table 1 were studied.
| Variant | Norm and term of fertilizer application | ||
|---|---|---|---|
| For pre sowing cultivation | The beginning of branching | The beginning of flowering | |
| 1 | - | - | - |
| 2 | Р30К30 | - | - |
| 3 | Р30К30 | N30 | - |
| 4 | Р30К30 | N30 | N30 |
| 5 | Р30К30 | N60 | - |
| 6 | N30 Р30К30 | - | - |
| 7 | N60 Р30К30 | - | - |
| 8 | N30 | - | - |
| 9 | N60 | - | - |
| 10 | N90 | - | - |
| 11 | N120 | - | - |
| 12 | - | N30 | N30 |
Table 1. Fertilizer options, application rates and deadlines.
Fertilizers were applied for sowing in the form of ammonium nitrate, simple granular superphosphate, potassium salt, and nitroammophoska (15:15:15); urea was used for top dressing during the growing season, during the active growth phase (the beginning of branching) and at the beginning of flowering. Factor B-inoculation: control without inoculation; inoculation with Rhizobofit, Rhizohumin, Bioinoculant BTU-L. Rhizobofit is a broad-spectrum inoculant for legumes and niche crops. Contains nodule bacteria of Mesorhizobium ciceri strain A-42. Rhizobophyte is a monostam preparation that includes highly active nodule bacteria that can compete with local strains of rhizobia and form an effective symbiosis. Rhizohumin for chickpeas is a complex inoculant that, in addition to strains of nitrogen-fixing bacteria, includes physiologically active compounds of biological origin in a concentration that optimally supports the vital activity of rhizobia and juvenile plants. The basis of the inoculant is strains of bacteria Mesorhizobium ciceri. The inoculant was created at the Institute of Agricultural Microbiology of the National Academy of Sciences.
Bioinoculant-BTU-L. Composition-symbiotic nodule bacteria that have a natural affinity for soy, peas, and other leguminous crops. Biologically active waste products of nodule bacteria (vitamins, phytohormones: heteroauxins, gibberellins), components of the nutrient medium (macro-, microelements). The minimum temperature when using the biological product is 10°C, the maximum is not higher than 30°C. Rhizobofit and Rhizohumin were used in the peat form, while the BTU inoculant was used as liquid. Seed treatment was carried out on the day of sowing using a liposam adhesive. The experiment used chickpea seeds of the Pamyat variety, which belongs to the Euro-Asian subspecies (subsp. eurasiaticum G. Pop.), type kabuli, variety bogemicoallutaceum G. Pop. (bohemian-allutaceum), the duration of the growing season is 90-95 days. Plant height 50-55 sm, attachment height of the lower beans 20-22 sm. Originator: Breeding and genetic Institute-National Center for Seed Science and Variety research the Plants have bush stature the National Academy of Sciences.
Chickpea cultivation agrotechnics are generally recognized for the conditions of the southern steppe of Ukraine. The previous crop, winter wheat for grain, sowing, in a continuous way with a row spacing of 15 cm; the seeding rate is 400-500 thousand seeds/ha. Harvesting was carried out using a Sampo-500 combine harvester in areas with grain samples selected for analysis; the grain weight was converted to standard humidity and 100% purity. The grain yield was recorded by direct weighing from the test site. During the branching and flowering phase, chickpea plants were selected to determine biometric indicators and calculate the number and mass of nodules. All records and observations were performed on two noncontiguous repetitions. Selection of experimental grain samples and determination of quality indicators were carried out according to standard methods: protein content-by infrared spectroscopy on the device Spectran-119m-DSTU 4117: 2007 (2007), weight of 1000 seeds-GOST 10842-89 (ISO 520-77) (2009), moisture content by the thermogravimetric method-GOST 13586.5-93 (1993).
Statistical processing of the obtained results was performed using Excel and Statistica softwares by the variance, correlation, regression, and graphical analysis (Ushkarenko et.all, 2008).
Results and Discussion
Our research shows that the timing of the the onset of phenological phases of chickpea growth and development and their duration were significantly dependent on the humidification conditions of the research year. Humidification conditions significantly affected the duration of the main interphase periods and the entire growing season. The duration of the period from sowing to germination was directly dependent on the temperature factor, the higher the sum of active temperatures, the faster the seedlings appeared (r=0.87), but the lack of moisture in combination with high temperatures delayed the emergence of the seedlings (coefficient of determination=0.85). For 2016-2018 years of research, shoots appeared: 2016 in 8 days, 2017 in 11 days, and 2018 in 17 days.
The weather conditions in 2016 contributed to the rapid emergence of seedlings, but extended the growing season of chickpeas in general. Excessively humid spring and early summer, when precipitation of the entire spring period exceeded the long-term average norm by 2-3 times, and in June by 13.8%, led to a significant lengthening of vegetation in general and the passage of individual plant growth phases (Table 2). The full ripeness phase occurred in 2016 after 119 days and in the hottest 2018 after 108 days.
| Year | Sowing-germination, days | Duration from full germination to phase, days | |||
|---|---|---|---|---|---|
| flowering | bean formation | seed formation | full ripeness | ||
| 2016 | 8 | 61 | 79 | 97 | 119 |
| 2017 | 11 | 58 | 76 | 96 | 115 |
| 2018 | 17 | 56 | 75 | 94 | 108 |
Table 2. Duration of interphase periods in chickpea otnogenesis (2016-2018).
The results of our observations on the importance of the influence of meteorological conditions on the duration of the growing season and individual phases of chickpeas are also confirmed by other researchers. During the research, there was practically no differentiation of biologics in terms of their effect on the passage of phases, growth, and development of plants. Only in the branching phase did the inoculants significantly increase the height of the plants compared to the control by an average of 13.5% over the years of research, but there is no significant difference between the inoculants themselves. Therefore, linear dimensions are given only for variants of fertilizer systems and years of research (Table 3, Figs. 2 and 3).
| variant | Fertilizer system | Plant height, cm | Regards control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | average for 3 years | sm | % | ||||
| 1 | control | 18.5 | 13.4 | 12.9 | 14.9 | 0 | 0 | ||
| 2 | Р30К30 | 20.6 | 15.3 | 13.8 | 16.6 | 1.7 | 11.4 | ||
| 3 | P30K30+N30 | 21.6 | 15.4 | 14.1 | 17.0 | 2.1 | 14.1 | ||
| 4 | P30K30+N30+N30 | 23.4 | 15.3 | 14.2 | 17.6 | 2.7 | 18.1 | ||
| 5 | P30K30+N60 | 24.4 | 14.8 | 13.6 | 17.6 | 2.7 | 18.1 | ||
| 6 | N30Р30К30 | 24.6 | 14.6 | 13.5 | 17.6 | 2.7 | 18.1 | ||
| 7 | N60Р30К30 | 25.3 | 14.3 | 13.8 | 17.8 | 2.9 | 19.5 | ||
| 8 | N30 | 21.3 | 15.4 | 14.1 | 16.9 | 2.0 | 13.4 | ||
| 9 | N60 | 24.2 | 15.8 | 14.7 | 18.2 | 3.3 | 22.1 | ||
| 10 | N90 | 25.4 | 14.9 | 14.4 | 18.2 | 3.3 | 22.1 | ||
| 11 | N120 | 24.8 | 15.1 | 14.6 | 18.2 | 3.3 | 22.1 | ||
| 12 | N30+N30 | 19.6 | 14.1 | 13.8 | 15.8 | 0.9 | 6.0 | ||
| LSD095 | 1,1 | 1.3 | 1.5 | ||||||
Table 3. Height of chickpea plants depends on the fertilizer system in the branching phase
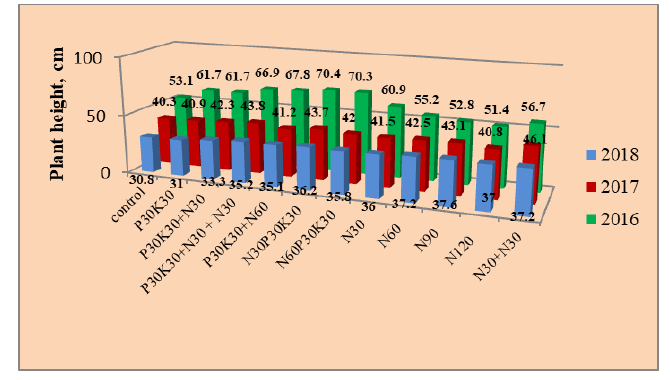
Fig 2: Height of chickpea plants at full ripeness by year and fertilizer variants.
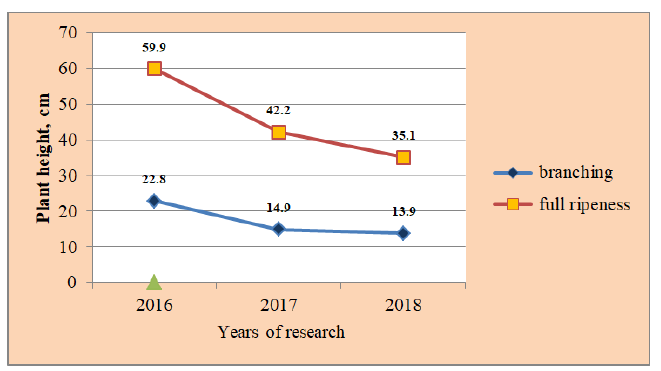
Fig 3: Average height of chickpea plants in research years.
It should only be noted that during the branching phase, inoculants also significantly increased the linear size of plant plants compared to the control by an average of 13.5% over the years of research, but there is no significant difference between the inoculants themselves. On average, over three years, fertilizer systems significantly (except Option 12) contributed to an increase in the height of chickpea plants during the branching phase: growth ranged from 11.4 to 22.1%. According to years of research, the degree of influence of fertilizers differed: In 2016, in all fertilizer variants, plant height growth was significant, both in relation to the control and the variant with the introduction of only phosphorus-potassium fertilizers; in almost all variants, except for variants with the application of full mineral fertilizer for pre-sowing cultivation, a significant increase in plant height was observed in relation only to the variant without fertilizers, and in 2018 a significant difference only between the control and the application of N60-120 during sowing.
The average height of chickpea plants in 2017 is less than in 2016 by 34.6% (branching) and 29.5% (full ripeness), and in 2018 by 39.5% and 41.4%, respectively (Fig. 3). Chickpea vegetation in the years of research was accompanied by soil and air droughts of various levels, which negatively affected nodule formation: Their number in one plant at its maximum did not exceed 7 pieces. Nodules on the roots of chickpeas began to form in the branching phase: in the absence of seed inoculation, an average of 1 nodule per two plants, when using inoculants-from 1.4 to 2 per plant. At this stage of chickpea plant development, the effect of inoculants on nodule formation processes is estimated at 17%, and already in the flowering phase, the number of nodules depended on inoculation at 37%. The average number of nodules per plant in the flowering phase, according to the variants of the rhizobia strains, ranged from 4.1 to 4.3 pieces, which is 1.6 to 1.7 times more than the control variant, and in the filling-the beginning phase of the phisiological ripeness-0.7 to 1.0 pieces versus 0.3 to control. In the future, we will consider the factors influence of the studied factors on the formation of nodules in the flowering phase.
On variants with the same amount of mineral fertilizers and the use of different biological preparations, the number of nodules formed on the same plant is very different (Fig. 4). Compared to the control, the best variant was with Rhizobofit seed treatment and the use of N30 foliar top dressing (during branching and at the beginning of flowering), where 6.8 pieces weighing 3.2 grams were formed per plant. However, the maximum mass of nodules in one plant was observed when Rhizohumin was used against the background of applying P30K30 (4.64 g) and its addition with foliar top dressing N30 (branching phase) 4.25 grams. In general, the mass of nodules by 59% is due to the foliar treatment of chickpea seeds and inoculants.
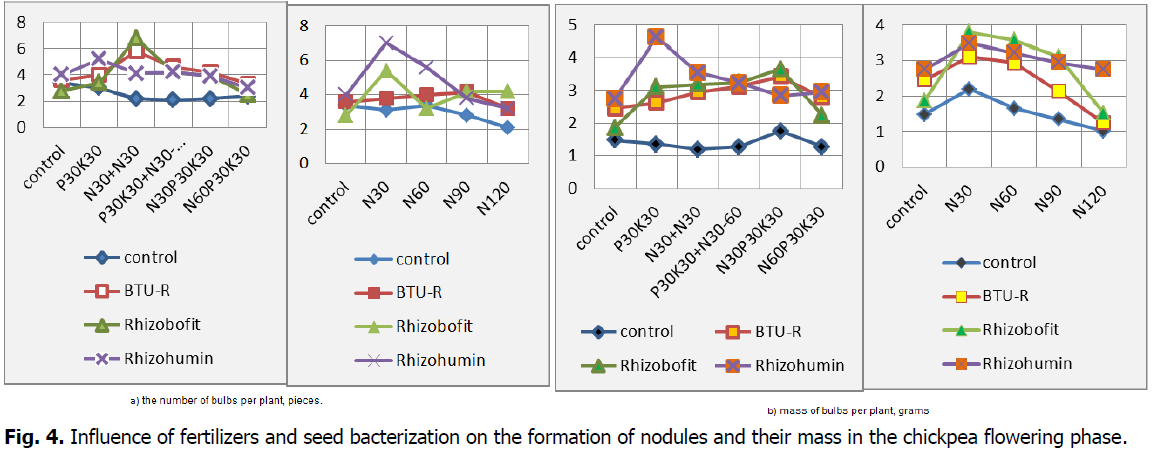
Fig 4: Influence of fertilizers and seed bacterization on the formation of nodules and their mass in the chickpea flowering phase.
It should be noted that an increase in the dose of near-sowing mineral nitrogen application from 30 kg/ha to 120 kg/ha led to inhibition of the nodule formation process. So, in the version without bacterization, the number of nodules, formed on one plant, decreased by 32.3%, with BTU-inoculant-by 15.8%, Rhizobofit-by 22.2%, and with Rhizohumin-by 54.3%, and their mass by 53.6%, 59.7%, 60.0% and 21.4%, respectively. Increasing the nitrogen content of a complete mineral fertilizer from N30 to N60 reduced the number of nodules by an average of 19.7% and their mass by 26.7%. At the same time, the preemergence application of N30P30K30 in comparison with P30K30 suppressed the process of nodules formation by a mathematically unreliable amount ( by 4.5%), and N60P30K30 significantly by 26.7%. The average mass of nodules in one plant during the flowering phase increased in the following rows: control (1.4 G) BTU-inoculant (2.8 g) Rhizobofit (3.0 g) Rhizohumin (3.2 g); similarly, the average mass of one nodule grew: 0.55 g 0.67 g 0.73 g 0.75 g.
Despite the high variability of data on the number and mass of nodules per plant over the years of research: from 30.6-35.6% (flowering phase) to 93.0-98.6%(branching), the positive effect of such a technique as pre-sowing inoculation of chickpea seeds with biological preparations (BTU-inoculant, Rhizobofit, Rhizohumin) is mathematically reliable or is at the level of reliability regardless. Many authors have obtained results that confirm the opinion about the negative impact of mineral fertilizers on the formation of nodules in the cultivation of chickpeas. Thus, according to the results of studies on Meadow-chernozem soils and southern chernozem, the application of mineral nitrogen fertilizers in doses of N30 and N60 reduced the number of nitrogen fixing nodules by 10-50%, their biomass by 2-6 times, nitrogen fixing activity by 2-18 times (Sichkar, Bushulyan, 2007; Bushulyan, Sichkar, 2011; Didovich et al., 2010). Herridge et al. (1995) studied the level of nitrogen fixation by chickpea culture at nitrogen rates: 0, 50 and 100 kg/ha. It was found that biologically fixed nitrogen ranged from 29 to 85 kg/ha and that soil nitrate nitrogen was used effectively by chickpeas, saving from 6 to 31 kg/ha and from 29 to 51 kg/ha in the second year of research. In our experiments on southern chernozem, it was shown that the application of N30P30K30 near sowing compared to P30K30 did not negatively affect the process of nodule formation (the difference was mathematically insignificant-4.5%), and N60P30K30 significantly reduced the number of nitrogen-fixing nodules by 30.2% and their biomass by 23.2%. At the same time, our observations established that the degree of negative effect of nitrogen doses growing from 30 to 120 kg/ha applied for pre-sowing cultivation depended not only on the nitrogen norm, but also on the inoculant, and, if on the variant without bacterization, the number of nodules formed in one plant decreased at N120 vs. N30 by 32.3%, then with BTU-inoculant and Rhizobofit-by 15.8% and 22.2%, respectively. The biomass of nodules decreased minimally in the variant of pre-seed bacterization with Rhizohumin (by 21.4%), and in other variants of bacterization and without it by more than 50%.
The main integral indicator of the action of a particular factor is the level of the formed crop. The complex effect of fertilizers and inoculants can not be traced very clearly: In the most favorable weather conditions of 2016, almost all combinations of factors significantly increased chickpea yield with the exception of Rhizohumin in N120, where the control crop was at the level of control without bacterization, but with the introduction of N120.
In 2017, in variant N120, the effectiveness of the BTU inoculant was at the level of the corresponding control, and Rhizobofit and Rhizohumin showed a significant decrease in productivity of 12.7% and 12.2%. A deeper decrease in the effectiveness of inoculants was observed in the variant with the introduction of N60P30K30, compared to p N30P30K30, which was (-21.6%) BTU; (-30.1) Rhizobofit and (-36.4%) Rhizohumin. During three years of research, high efficiency of inoculants was observed in the variant of double feeding with mineral nitrogen: in 2016, Rhizobofit contributed to an increase of 24.5%, in 2017-BTU inoculant by 17.5% and in 2018 by the same amount, but Rhizohumin. On average, over three years, when using the BTU inoculant against the background of N30+N30, the chickpea yield increased by 13.9%, with Rhizobofit by 11.8 and with Rhizohumin by 4%. It should be noted that the effectiveness of the BTU-inoculant was more stable than that of Rhizobofit and Rhizohumin. Yield gains during chickpea seed bacterization with BTU-inoculant are reliable for three years of research; Rhizobofit-two years out of three and Rhizohumin-one year out of three (Table 4).
| Inoculant | 2016 | 2017 | 2018 | average | Regards control | |
|---|---|---|---|---|---|---|
| t/ga | % | |||||
| without inoculation | 2.88 | 2.29 | 1.28 | 2.15 | 0 | 0 |
| BTU-R | 3.56* | 2.50* | 1.47* | 2.51* | 0.36 | 16,7 |
| Rhizobofit | 3.11* | 2.37 | 1.45* | 2.31 | 0.16 | 7,4 |
| Rhizohumin | 2.99 | 2.34 | 1.40* | 2.21 | 0,06 | 2,8 |
| LSD095 | 0.12 | 0.11 | 0.06 | |||
Table 4. Effect of inoculants on chickpea grain yield.
If we consider the effect of different doses of nitrogen fertilizers (Fig. 5) and full mineral content (Fig. 6), then it was similar to the effect of the Complement Factor. In 2016, all fertilizers gave a significant increase (N120-on the verge of reliability), in 2017, there was a significant decrease in the yield of chickpea grain when N120 and full mineral fertilizer were applied for sowing-by 19.4%, 11.3% and 25.5%, according to the non-fertilized version.
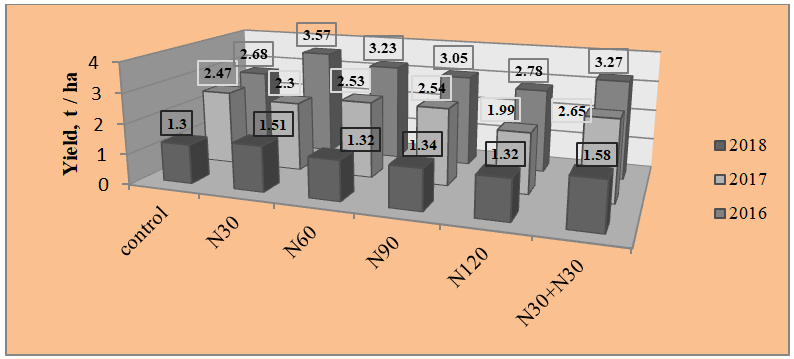
Fig 5: Effect of nitrogen fertilizer dose on chickpea grain yield, t/ha.
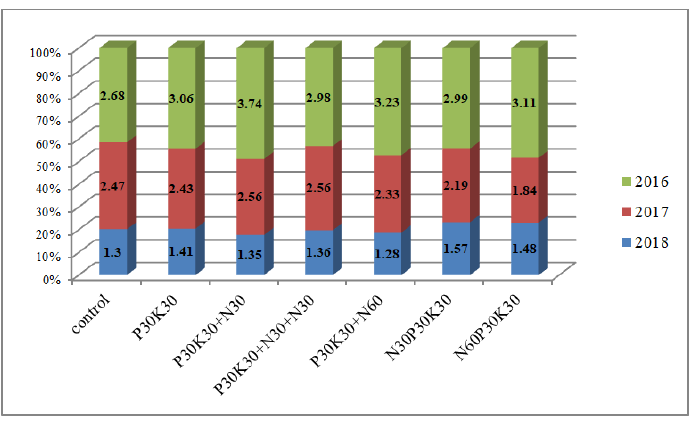
Fig 6: Chickpea grain yield by full mineral fertilizer and the share of each year in the average productivity of chickpea crop.
Thus, according to the effect on productivity, chickpeas are isolated from inoculants-BTU inoculant, from fertilizer systems-variants where the minimum nitrogen rate is introduced, and with the complex action of inoculants, the fertilizer system is the bacterization of seeds with BTU-inoculant or rhizobofit and the introduction of N30+N30 (the beginning of branching and the beginning of flowering). If the soil has low available phosphorus and potassium, we recommend applying P30K30 during sowing and a single foliar top dressing N30.
According to Tomnicki (2009, 2012), with a low content of nitrate nitrogen in the soil, mobile medium compounds of phosphorus and potassium, it is recommended to apply mineral fertilizers at a dose of N30P30K30, which in some aspects is not disputed by the results of our research. In other studies, it is noted that in poor soils, the introduction of N30-45 for pre-sowing cultivation has a positive effect (Cinerem, 2011).
Influence of fertilizer systems and inoculants on chickpea grain quality indicators
The mass of 1000 chickpea grains ranged on average from 234.6 to 260.2 grams. Mineral fertilizers are a powerful factor in changing the size of chickpea grain. The growing of chickpeas on a natural background of fertility provided the formation of the smallest mass of 1000 grains, which was, on average, 203.3 grams in the absence of inoculation and ranged in fertilizer from 220.7 to 234.6 G. In the absence of pre-sowing inoculation, fertilizer systems significantly improved the caliber of chickpea seeds, from 8.4 to 15.4%. Among the inoculants, Rhizobofit can be distinguished by its effect on the mass of 1000 grains: the increase was from 3.2 to 12.0%, when using a BTU inoculant, the grain mass also increased, but by a relatively smaller amount-from 2.7 to 13.5%. Rhizohumin had the least effect on the mass of 1000 seeds: an increase in the mass of compositions from 0.7 to 9.1%, on some variants (N90-120 and P30K30+N30+N30)-there was a tendency to decrease the grain caliber. The introduction of mineral nitrogen during the growing season, both without inoculation and in combination with bacterization, made it possible to increase the mass of 1000 seeds from 15% (without inoculants) to 9.1-13.5%. Inoculants by effect on grain mass can be arranged in descending order of effect on this indicator B as follows: BTU-inoculant (13.5%) Rhizobofit (12.0%) Rhizohumin (9.1%).
The influence of both fertilizer systems and the complex action of the fertilizer system and presown seed treatment is more obvious on the protein concentration in chickpea grain than on its main physical parameter. The protein content of the grain increased significantly in all variants of the experiment (Fig.7).
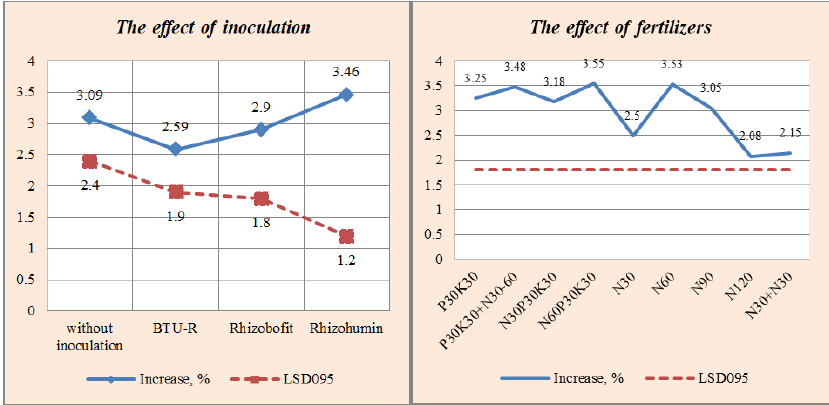
Fig 7: Increase in protein content in chickpea grain (% to control).
Conclusion
Based on the results of the experimental studies carried out during 2016-2018, the following scientific generalizations and conclusions can be formulated.
According to the totality of characteristics, the conclusion is distinguished: application of P30K30 for pre-sowing cultivation+N30 in the branching phase in combination with seed bacterization with BTU-L; the increase in yield was 1.50 t/ha or 16.7 kg per unit of active substance of fertilizers compared to the variant without fertilizers.
On average, according to seed bacterization, compared to P30K30, a single top dressing of chickpea plants in the branching phase with mineral nitrogen at a dose of 30 kg/ha provided a return of a unit of active nitrogen substance-8.3 kg.
In fields with an average and higher content of available forms of phosphorus and potassium, double application is effective: N30 in the branching phase+N30 at the beginning of the bluff. Pre-sow inoculation of chickpea seeds with BTU-inoculant and Rhizobofit preparations is combined with them. The increase in yield is 0.99-0.89 t/ha, the return on a unit of fertilizers with chickpea grain is 16.5-14.8 kg, and on average for bacterization, the return is 5.8 kg.
Increasing the dose of pre-sowing application from N30 to N120 suppresses the process of nodule formation without seed bacterization by 32.3%, using inoculants-from 15.8% to 54.3%, and their mass by 53.6% and from 21.4% to 60.0%, respectively.
Increasing the rate of nitrogen in the composition of a complete mineral fertilizer from N30 to N60 reduced the number of nodules by an average of 19.7% and their mass by 26.7%, but compared to the introduction of P30K30, N30P30K30 did not significantly (by 4.5%) reduce these indicators and N60P30K30 significantly by 26.7%.
Fertilization significantly increased the protein content of chickpea grain. The mass of 1000 seeds was formed under the influence of both factors, while the share of exposure to inoculant ranged from 20 to 31%.
References
Akinerbem, F. (2011). Praktiki o vyrashivanii nuta. Zerno, 2:60-64 (in Russian).
Balashov, A.V. (2011). Osobennosti selekcii, semenovodstva i tehnologii vozdelyvaniya sortov nuta, adaptirovannyh k zasushlivym usloviyam Nizhnego Povolzhya. Thesis of Doctoral Dissertation. Volgograd State Agrarian Academy. Volgograd (in Russian).
Balashova, N.N. (2003). Mirovye tendencii proizvodstva i potrebleniya nuta. Zernovoe Hozyajstvo, 8:5-8 (in Russian).
Burykina, S.I., Tsurkan, O.I. (2020). Trends of modern changes in the agro-climatic situation on the territory of the steppe Chernozem zone of the South of Ukraine. Tavrijskij Naukovij Visnik, 111:29-43.
Bushulyan, O., Sichkar, V. (2011). Nut kak novyj kozyr sevooborota. Zerno, 2:54-58 (in Russian).
Bushulyan, O.V., Sichkar, V.I. (2009). Nut: genetika, selekciya, nasinnictvo, tehnologiya viroshuvannya: monografiya. Odesa: SGI-NCNS (in Ukrainian).
Bushulyan, O.V. (2003). Nut na yuge Ukrainy: sostoyanie i perspektivy. Visnik agrarnoyi nauki Prichornomor’ya, 2:115-119 (in Russian).
Bushulyan, O.V., Sichkar, V.I., Babayanc, O.V. (2012). Integrovana sistema zahistu nutu vid bur᾽yaniv i hvorob: metodichni rekomendaciyi. Odesa: SGI-NCNS (in Ukrainian).
Chen, C., Jackson, G., Neill, K., Miller, J. (2006). Spring pea, lentil and chickpea response to phosphorus fertilizer. Fertilizer, 38:29-30.
Didovich, S.V. (2010). Biologizaciya agrotehnologii vyrashivaniya nuta: rekomendacii po effektivnomu primeneniyu mikrobnyh preparatov. Simferopol: ChP Eremina V.G. (in Russian).
Didovich, S.V., Portyanko, S.I., Didovich, O.M., Tolkachev, M.Z. (2006). Vpliv mineralnogo azotu na efektivnist simbiozu nutu. Silskogospodarska mikrobiologiya: Mizhvid Temat Nauk Zb, 3:43-54 (in Ukrainian).
Didovich, S.V., Tolkachov, M.Z., Shabanov, E.A., Shigorcova, O.L. (2005). Efektivnist nitraginizaciyi nutu. Agroekologichnij Zhurnal, 2:48-51 (in Ukrainian).
Dospehov, B.A. (1971). Planirovanie polevogo opyta i statisticheskaya obrabotka ego dannyah. Moscow: Kolos (in Russian).
Dospehov, B.A. (1985). Metodika polevogo opyta. Moscow: Agropromizdat (in Russian).
Draganchuk, M. (2011). Nut: agrotehnika vyrashivaniya. Fermerske gospodarstvo, 35:18 (in Russian).
DSTU. (2007). Zerno ta produkti jogo pererobki. Viznachennya pokaznikiv yakosti metodom infrachervonoyi spektroskopiyi. Kyiv: Derzhspozhivstandart Ukrayini (in Ukrainian).
Gamayunova, V.V., Tomnickij, A.V. (2013). Vpliv mineralnih dobriv na pozhivnij rezhim temno-kashtanovogo gruntu ta vrozhajnist nutu. Visnik Sumskogo nacionalnogo agrarnogo universitetu. Seriies ”Agronomiya i biologiya”, 3:67-71 (in Ukrainian).
Germanceva, N.I., Filatov, A.N., Kalinina, G.V., Selezneva, T.V. (2002). Novyj sort nuta Zavolzhskij i tehnologiya ego vozdelyvaniya. Zernovoe Hozyajstvo, 4:9-11 (in Russian).
Gorodnij, M.G. (1981). Roslinnictvo. Laboratorno-praktichni zanyattya. Kyiv: Visha shkola (in Ukrainian).
Goroh, nut-eda bednyakov i faraonov. Niva: Vseukrayinskij Shomisyachnij Agrarnij Zhurnal, 11:38-42 (in Russian).
GOST. (2009). Zerno zernovyh i bobovyh kultur i semyan maslichnyh kultur. Metod opredeleniya massy 1000 zeren ili 1000 semyan. Moscow: Standartinform (in Russian).
GOST. (1993). Zerno. Metod opredeleniya vlazhnosti. Mezhgosudarstvennyj sonet po standartizacii, metrologi i sertifikacii. Minsk (in Russian).
Herridge, D.F. (1995). Chickpea increases soil-N fertility in cereal systems through nitrate sparing and N2 fixation. Soil Biology and Biochemistry, 27:545-551.
Kolesnikov, A. (1997). Excel dlya polzovatelya. Analiz dannyh. Delovaya grafika. Rabota v seti. Kiev: Torgovo-izdatelskoe byuro BHV (in Russian).
Kulbida, M.I., Yelistratova, L.O., Barabash, M.B. (2013). Suchasnij stan klimatu Ukrayini. Problemi Ohoroni Navkolishnogo Prirodnogo Seredovisha Ta Ekologichnoyi Bezpeki, 35:118-130.
Kulinich, O. (2005). Vnosimo azot z bobovimi. Propoziciya, 5:50 (in Ukrainian).
Lavrenko, N.M. (2015). Urozhajnist ta yakist zerna nutu zalezhno vid tehnologichnih prijomiv viroshuvannya za riznih umov zvolozhennya. Thesis of Doctoral Dissertation. Kherson State Agrarian University. Kherson (in Ukrainian).
Lihochvor, V.V., Petrichenko, V.F., Ivashuk, P.V. (2008). Zernovirobnictvo. Lviv: Ukrayinski tehnologiyi (in Ukrainian).
Lihvar, D.F. (1964). Zernovi bobovi kulturi. Kiyiv: Urozhaj (in Ukrainian).
Mihajlenko, L.P. (2005). Formuvannya produkcijnogo procesu zernobobovih kultur pid vplivom pogodnih i tehnologichnih faktoriv v pivnichnomu Stepu. Thesis of Doctoral Dissertation. Dnipropetrovsk (in Ukrainian).
Nezar, H., Nezar, S., Haddad, N., Alqudah, A.M. (2009). Yield potential evaluation in chickpea genotypes under late terminal drought in relation to the length of reproductive stage. Italian Journal of Agronomy: Riv. Agron, 3:111-117.
Pipie, F. (1983). Tehnologia de cultura a nautului. Productia Vegetala Horticultura, 35:28-30.
Podgornyj, P.I. (1957). Rastenievodstvo. Moskva: Gosselhozizdat (in Russian).
Polikarpov, V.L. (2003). Osobennosti tehnologii vyrashivaniya semyan nuta v yuzhnoj lesostepi CChR. Thesis of Doctoral Dissertation. Voronezh State Agrarian University. Voronezh (in Russian).
Polovij, A.M., Bozhko, L.Yu. (2015). Vpliv klimatichnih zmin na rezhim zvolozhennya vegetacijnogo periodu v Ukrayini. Ukrayinskij Gidrometeorologichnij Zhurnal, 16:128-139 (in Ukrainian).
Roata, M. (1983). Thehnologia de cultura a nautului aplicata la C.A.P. “N. Balcescu”- judetul Teleorman. Production Vegetation Cereale Plante Tehn, 35:54-55.
Rozvadovskij, A.M. (1990). Zernobobovi kulturi v intensivnomu zemlerobstvi. Kyiv: Urozhaj (in Ukrainian).
Sherbakova, O.M. (2015). Produktivnist nutu ta aktivnist bobovo-rizobialnoyi sistemi roslin za peredposivnoyi obrobki nasinnya v Pravoberezhnomu Lisostepu Ukrayini. Thesis of Doctoral Dissertation. Kyiv (in Ukrainian).
Shyurova, N.A. (2004). Produktivnost i simbioticheskaya aktivnost nuta v zavisimosti ot priemov vyrashivaniya v stepnoj i suhostepnoj zonah Saratovskoj oblasti. Thesis of Doctoral Dissertation. Saratov (in Russian).
Sichkar, V.I., Bushulyan, O.V. (2007). Nut Botanichna harakteristika, biologichni osoblivosti, agrotehnika ta novi sorti. Odesa: SGI-NAC NAIS (in Ukrainian).
Sichkar, V.I. (2004). Rol zernobobovih kultur u virishenni bilkovoyi problemi v krayini. Kormi i Kormovirobnictvo, 53:110-115 (in Ukrainian).
Sitova, V.M., Adamenkob T.I. (2011). Agroklimatichnij dovidnik po Odeskij oblasti. Odesa: Astroprint (in Ukrainian).
Stepanenko, S.M., Polovij, A.M., Dem’yanyuk, O.S. (2014). Zmina rezhimu opadiv v Ukrayini. Agroekol Zhurnal, 2:10-16 (in Ukrainian).
Stolyarov, O.V., Demchenko, N.I. (2002). Vliyanie norm vyseva i sposobov poseva na urozhaj i kachestvo semyan nuta v stepnoj chasti CChR. Information Listok, Voronezh (in Russian).
Tanchik, S.P. (2008). Tehnologiyi virobnictva produkciyi roslinnictva. Kiyiv: Vidavnichij Dim Slovo (in Ukrainian).
Tolkachev, N.Z., Didovich, S.V. (2003). Vliyanie inokulyacii semyan biopreparatami mikrobov-antagonistami fitopatogenov na simbioz rastenij s Rhizobium cicero. Biologichni nauki i problemi roslinnictva: Zb Nauk Prac Umanskogo derzh. Agroun-tu. Specvipusk, pp:287-291 (in Russian).
Tolkachev, N.Z., Didovich, S.V., Shabanov, E.A. (2003). Effektivnost nitraginizacii nuta v Krymu. Zb nauk prac Luganskogo nac. Agrar un-tu, 30:62-66 (in Russian).
Tomnickij, A.V. (2009). Vpliv sistem zhivlennya na formuvannya produktivnosti nutu v nepolivnih umovah pivdnya Ukrayini. Proceeding All-Ukrainian Science Conference. “Problemi ta perspektivi vedennya zemlerobstva v posushlivij zoni Stepu Ukrayini”, pp:78-79 (in Ukrainian).
Tomnickij, A.V. (2012). Efektivnist mineralnih dobriv pri viroshuvanni nutu v umovah pivdennogo stepu Ukrayini. Thesis of Doctoral Dissertation. National Scieitific Center “Institut gruntoznavstva ta agrohimiyi im. O.N. Sokolovskogo”. Kharkiv (in Ukrainian).
Ushkarenko, V.O., Nikishenko, V.L., Goloborodko, S.P., Kokovihin, S.V. (2008). Dispersijnij i korelyacijnij analiz rezultativ polovih doslidiv. Kherson (in Ukrainian).
Zinchenko, O.I., Salatenko, V.N., Bilonozhko, M.A. (2003). Roslinnictvo. Kyiv: Agrarna osvita (in Ukrainian).
Author Info
S. Burykina1*, A. Kryvenko1*, I. Gulyaeva2, V. Gamayunova3 and A. Shepel42Odessa state Agrarian University, 13 Panteleimonovskaya St, 65000, Odessa, Ukraine
3Nikolaev National Agrarian University, 9 Georgiy Gongadze St, Nikolaev, 54000, Ukraine
4Kherson State Agrarian and Economic University, 23 Stritenskaya St, Kherson, 73006, Ukraine
Citation: Burykina, S., Kryvenko, A., Gulyaeva, I., Gamayunova, V., Shepel, A. (2021). Influence of mineral fertilizers and inoculants on the yield and quality of chickpea grain. Ukrainian Journal of Ecology 11 (9), 150-158.
Received: 02-Nov-2021 Accepted: 30-Nov-2021 Published: 08-Dec-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
