Research Article - (2022) Volume 12, Issue 2
Infectious risks of biofilms on medical implants
D. Oukid*Abstract
Within a biofilm, there is normally a notion of spatial structuring, the microbes live there in 3D and are called adherent cells. Biofilms formed on medical implants pose a serious public health problem and interfere with the functioning of various medical implants as they present a microbial biological fortress. This way of life provides better resistance to environmental aggressions, whether biotic or abiotic. Moreover, the catheters used today at the hospital level, especially the peripheral venous catheters, are at high risk of biofilm formation and thus patient infection. This high risk is due to many reasons, including the chemical composition and the morphology of the implant surface. To protect these medical devices against pollution and infectious risks and thus avoid (reduce) hospital-acquired infections, we should understand all biofilm-surface interactions from a microscopic, chemical and metabolic point of view. It is therefore in this context that this research project finds its meaning, the general objective is to deepen our knowledge of the phenomena governing the structuring of biofilms for the control and management of their activities.
Keywords
Biofilms, Risks, Implants, Interaction, Surface.
Introduction
Biofilms are microorganism communities that grow on different types of surfaces. Although they are beneficial (useful) in most natural environments, the biofilms that grow on medical implants, especially in the case of chronic injuries and infections, constitute a real medical dilemma as they cause many nosocomial infections. Currently, medical devices are very important in medicine (treatment) as in most periods of health care medical implants such as vascular catheters and bladder probes are installed in the patient's body to facilitate medication (injecting medication into his blood, taking blood samples, etc.). Unfortunately, they-medical implants- are associated with very significant infection risk, as 60 percent of medical care infections are closely related to these devices, which represent a suitable surface for the growth of biofilms and microbes (Florence, E., et al., 2010). It is therefore in this context that this research project finds its meaning. The general objective of this research is to deepen our knowledge of the risks of biofilm infection associated with medical implants, as well as the various phenomena controlling biofilm formation on them, to reach solutions (methods) that allow managing their activities and thus reducing nosocomial infections.
Biofilm formation on medical implants
Despite the continuous technical and medical development, microbial contamination (pollution) and infection in medical implants still pose serious risks and threats. Biofilms formed on biomedical implants lead to their damage and thus their failure. Medical device-related infections account for more than 60 percent of hospital infections (Bryers, J.D., 2008), which is a worrying percentage. The risk of this type of infection increases in patients with serious illnesses (Darouiche, R.O., et al., 1998).
Moreover, bacterial biofilms have the ability to colonize many different types of medical devices resulting in the spread of pathogens that may lead to serious infections or even death of the patient (Hall-Stoodley, L., et al., 2004).
There are specific bacterial species that contribute to the formation of biofilms on intravenous catheters, some of them are Gram-positive (Enterococcus faecalis, S. aureus, S. epidermidis) and some are Gram-negative (E. coli, Klebsiella pneumoniae, Proteus mirabilis, P. aeruginosa) (Davey, M.E., et al., 2000). Some of these species are found naturally on human tissues, such as the skin and intestines.
Catheter-associated biofilm infection is characterized by resistance to the defense mechanisms of the immune system and to various antibiotics currently in use, making it difficult to treat. It is worth noting that bacterial infections caused by biofilms pollution are frequent despite the preventive measures taken at the hospital level.
Due to the difficulty of treating this infection, preventing biofilm formation on these medical devices is the best solution to suppress it.
Medical complications related to biofilms
In order to classify the infection resulting from microbial pollution of medical implants, as biofilm-associated infection, the following criteria and conditions must be met (Parsek, M.R., et al., 2003):
• Bacteria should be adhered (attached) to a surface or depend on the substrate of the medical implant;
• Bacteria should be organized in communities and contained in a matrix composed of bacteria or host elements;
• Primary infection (initiation of infection) must be confined to a specific part of the patient's body (medical implant site);
• l’infection doit résister à l’antibiothérapie, bien que les bactéries testées en phase planctonique y soient sensibles.
The biofilm infection may evolve from a medical implants infection to a serious chronic infection. Fig. 1 below summarizes the various types of infections associated with biofilm.
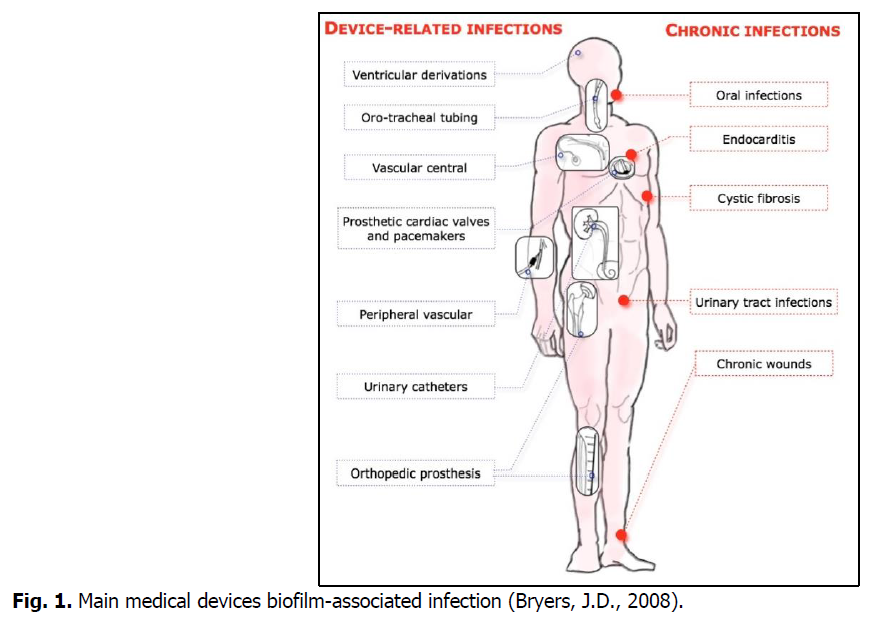
Fig 1. Main medical devices biofilm-associated infection (Bryers, J.D., 2008).
Materials and Methods
In this research, we focus on studying the biofilm associated with peripheral vascular catheters. Samples were taken from different units of "Dali Abdelkader" Hospital, located in the city of Ghriss, the state of Mascara, Algeria.
Samples preparation
The samples were placed directly in sterile boxes, after being removed from the patients' arms, by paramedical personnel. They were transferred without delay to the laboratory, where 20 ml of sterile physiological water was added to each boxes before being shaken using the Vibrator Vortex (Fig. 2).

Fig 2. Samples preparation.
The goal of this process is to collect the largest possible amount of microbes adhered to the catheters (samples).
Quantification of microorganisms
Before going into the isolation process, it was necessary to quantify microbes adhered to catheters, which could lead to biofilm formation. To achieve this, 100 μl of the prepared samples solution were taken and spread in Agar medium. Then the cultures were incubated at 37°C for 24 hours (Two culture media for each sample). The chart below provides a detailed explanation of this process (Fig. 3).
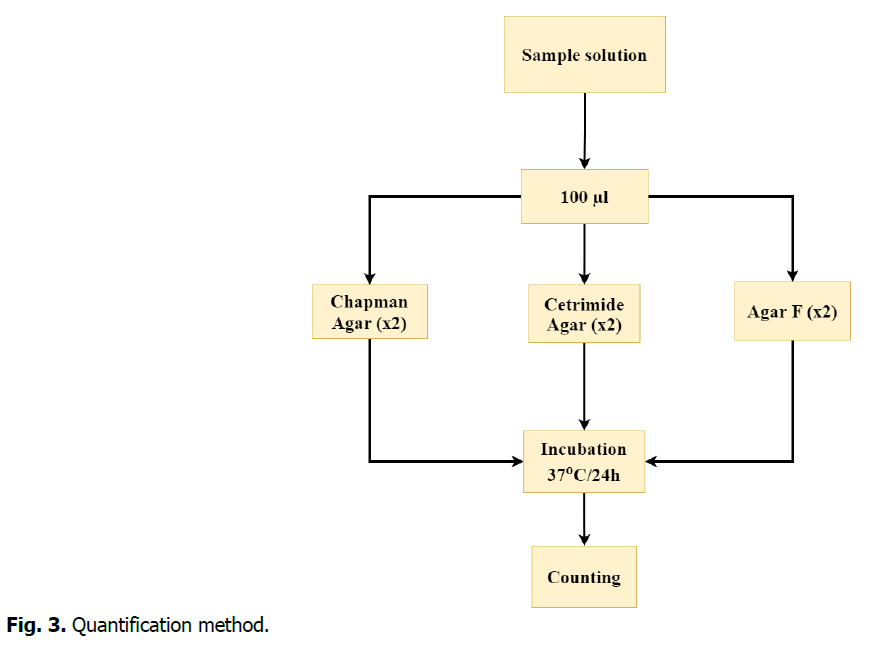
Fig 3. Quantification method.
Isolation and purification
The objective of this process is to obtain pure microbial cultures. To isolate microbes from each other, especially bacteria, selective culture media was used, such as Chapman's Agar and Agar F. To ensure that pure strains were obtained, the streak plate method was used (Fig. 4).
Fig 4. Streak plate method.
Results and Discussion
The results of quantification were somewhat different depending on the medical unit to which the sample belonged. Table 1 highlights the results of microbial quantification obtained by type of culture medium and the target medical unit.
| Medical unit | ENT 1 | ENT 2 | Medicine man 1 |
|---|---|---|---|
| CFUs | 40 | Cancelled (>330 colonies were counted.) | 6.3 × 103 |
Table 1. Quantification results.
The numbers we obtain are significant and cannot be neglected. The presence of this number of microbes, which the results of the identification methods showed to be one of the most prominent species responsible for biofilm formation on catheters, is worrisome and the reasons that led to the presence of this number of microbes on this sensitive medical device must be searched.
On the other hand, the results of isolation and purification, as well as identification, revealed the presence of the following species of bacteria on the studied samples: S. aureus, S. epidermidis and P. aeruginosa
These species have the ability to form biofilms on medical devices and thus cause damage to them on the one hand, and nosocomial infections on the other hand, which threatens the health of the patient.
The isolated strains will undergo a biofilm formation test in order to reach a method that allows the prevention of the risk of biofilm formation on catheters.
Reasons that increase the risk of biofilm in the target hospital
The factors that led to these results can be summarized in the following points:
• The deteriorating condition of the hospital, which is considered an expired building.
• The negligence of nurses and paramedics in respecting hygiene standards, such as wearing medical gloves.
• Peripheral catheters are used for longer than the recommended period (Sometimes more than 10 days).
• Frequent contact of the patient, and sometimes visitors, to the peripheral catheters without sterilizing the hands, which increases the risk of biofilm formation.
Preventive measures
The application of maximum hygiene measures in the operative context is not specifically an antibiofilm measure. However, the limitation of the risk of contamination of the surgical site and the strict application of the recommendations concerning the handling of the devices limit the risk of initial bacterial adhesion. When placing central catheters, the application of surgical asepsis (maximum sterile barrier precautions) has been shown to be effective in reducing the risk of infection associated with these devices (O’Grady, N.P., et al., 2011).
Conclusion
Implant-associated microbial infection poses a serious threat and remains a major cause of failure of biomedical implants. Rigorous and extensive application of hygiene standards is essential to reduce this risk. Research efforts should be intensified on novel biofilm control strategies such as innovative surface modification approaches to prevent bacterial infections in medical implants in order to provide a unique shield against biofilm infection.
References
Florence, E., Bernard, P., Brigitte, C.B. (2010). Risques infectieux associés aux dispositifs médicaux invasifs. Revue Francophone des Laboratoires.
Bryers, J.D. (2008). Medical biofilms. Biotechnology and Bioengeering, 100:1-18.
Darouiche, R.O., Farmer, J., Chaput, C. (1998). Anti infective efficacy of antiseptic-coated intramedullary nails. Journal of Bone and Joint Surgery, 80:1336-1340.
Hall-Stoodley, L., Costerton, J.W., Stoodley, P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology, 2:95-108.
Davey, M.E., O’toole, G.A. (2000). Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Review, 64:847-867.
Parsek, M.R., Singh, P.K. (2003). Bacterial biofilms: an emerging link to disease pathogenesis. Annual Review of Microbiology, 57:677-701.
O’Grady, N.P., Alexander, M., Burns, L.A. (2011). Guidelines for the prevention of intravascular catheter related infections. Clinical Infection Diseases, 52:e162-93.
Author Info
D. Oukid*Citation: Oukid, D. (2022). Infectious risks of biofilms on medical implants. Ukrainian Journal of Ecology. 12:64-67.
Received: 08-Feb-2022, Manuscript No. UJE-22-53928; Accepted: 03-Mar-2022, Pre QC No. P-53928; Editor assigned: 10-Feb-2022, Pre QC No. P-53928; Reviewed: 21-Feb-2022, QC No. Q-53928; Revised: 26-Feb-2022, Manuscript No. R-53928; Published: 10-Mar-2022, DOI: 10.15421/2022_346
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
