Research Article - (2021) Volume 0, Issue 0
Immunohistochemical characterisation of lymphoid subpopulations and CD34+ cells in the lymphoid tissue of rabbit appendix
Khomych, V.T., Fedorenko, O.V.*, Mazurkevych, T.A. and Dyshlyuk, N.VAbstract
Objectives: The cecal appendix is present in 50 mammalian species, including the domestic rabbit. According to current data, it carries out many functions, the main of which is its role as a component of peripheral (secondary) organs of hemo and lymphopoiesis. In addition, some studies reported the appendix may function as an equivalent of the avian bursa of Fabricius and be a source for the development of B cells in mammals.
For the determination of the cecal appendix's exact functions as an organ of hemo and lymphopoiesis, immunohistochemical studies of its lymphoid tissue are necessary. They enable the identification of its cellular composition and analysis of its role in hematopoiesis. Therefore, the aim of our study was to establish the content and localization of hematopoietic stem cells and lymphoid subpopulations in the domestic rabbit cecal appendix.
Materials and Methods: Cecal appendix tissue samples were collected from four 4-months-old Pannon White male rabbits. For immunohistochemical studies, monoclonal antibodies CD3, CD10, CD20, and CD34 (DAKO, Denmark) were used.
Results: We found that lymphoid tissue of the rabbit appendix contains CD34+ cells. They are predominantly in the germinal centers of secondary lymphoid nodules and also appear in the domes of lymphoid nodules and diffuse lymphoid tissue. We found a few CD34+ cells in the walls of the crypts in the mucosa. CD20+ cells (B cells) predominated among the lymphoid subpopulations in the nodular form of lymphoid tissue. It also contained many CD10+ cells (precursors of T cells and B cells), which were mainly concentrated in the germinal centers of secondary lymphoid nodules. In the diffuse form of lymphoid tissue, the vast majority of cells expressed the CD3 marker (T cells).
Conclusions: The presence of CD34+ cells in the lymphoid tissue of the rabbit appendix may indicate hematopoietic stem cells.
Keywords
Rabbit, CD markers, immunohistochemistry, appendix, cecum.
Introduction
Rabbits are the source of dietary meat and fur. They are also used in laboratory studies as animal models, because their tissues and organs are more similar to humans compared to rodents (Mage et al., 2016) (O'Malley, 2005). In particular, many similarities were found between gut-associated lymphoid tissue of rabbits and humans. Many comparisons have been made between rabbit and human cecal appendix, based on the development of antibodies that take place in them (Haines et al., 2016). The appendix was found in 50 of 361 existing mammalian species (Smith et al., 2013). Rabbit is the only animal species frequently used in laboratory studies that has an appendix (Girard-Madoux et al., 2018) (Stan, 2014).
50% of rabbit lymphoid tissue is concentrated in appendix and sacculus rotundus (Hulls, 2015). The rabbit cecum serves as a fermentation repository, where microflora breaks down fiber and proteins to short-chain fatty acids (O’Malley, 2005) (Hulls, 2015). Therefore, the immune system of the large intestine must control the commensal microbiota, preventing the immune response that could lead to its removal (Kuper et al., 2017).
The main function of appendix is to protect intestine and its microflora from pathogenic agents. It also produces amylase, lipase and hormones that are involved in the contraction of the intestinal sphincters and regulate its peristalsis (Aminova, 2019). The appendix releases bicarbonate ions into the lumen of the cecum, which act as a buffer for volatile fatty acids formed during fermentation in the intestine (Davies & Rees Davies, 2003) (Varga & Harcourt-Brown, 2014) (O’Malley, 2005). It is one of the main sites for the production of IgA, which is necessary for the regulation of composition and density of intestinal microbiota. New lymphocytes are formed more intensively in rabbit appendix compared to other organs of lymphopoiesis. Lymphoid nodules in the appendix may play important role in obtaining antigenic information about the intestinal content and its distribution to cells responsible for the production of antibodies in other organs (Hanson & Lanning, 2008) (Pospisil et al., 2006). Furthermore, the shape and location of appendix indicate that it may be a niche for commensal bacteria. It contains a lot of biofilms, from which bacteria are shed into the intestinal lumen. The appendix can restore the microbiota of the large intestine after diarrhea (Girard-Madoux et al., 2018) (Kooij et al., 2016).
As early as the 1960s, it was suggested (Archer et al., 1963) that the rabbit appendix might be the equivalent of the avian bursa of Fabricius. This hypothesis was supported by studies on rabbits after appendectomy, which showed lower antibody titers in response to antigen challenge, fewer circulating lymphocytes in peripheral blood, and reduced lymphopoiesis in peripheral lymphoid tissue. The decrease in the B cell pool could not be explained by the loss of B cells located in the appendix during its removal alone (Weinstein et al., 1994) (De Coppi et al., 2006). Similarly, bursectomy leads to a significant decrease in the number of plasma cells and the humoral response (Dasso et al., 2000). But other researchers have not obtained similar results after appendectomy in 3- week-old rabbits, suggesting that the appendix may play an important role in the development of the primary antibody repertoire in the neonatal period (De Coppi et al., 2006).
The rabbit appendix, as well as the ileal Peyer's patches in pigs and cattle, is considered to have features of the central (primary) organs of hemo- and lymphopoiesis (G.A. Parker & Makori, 2018) (Dasso et al., 2000) (D. Lanning et al., 2000). It is the site of development, proliferation and differentiation of B cells and diversification of the primary antibody repertoire (Hanson & Lanning, 2008) (Pospisil et al., 2006). However, according to other researchers appendix belongs to the secondary lymphoid organs. If we consider the appendix as an analogue of the primary lymphoid organ for B cells, then in contrast to the thymus, into which antigens do not penetrate, the appendix contains a massive portion of the microbial antigen and the specific gut flora is necessary for diversification of the antibody repertoire (Butler & Sinkora, 2013) (D. Lanning et al., 2000).
Other researchers suggested the possibility of the existence of a period when appendix simultaneously functions as a central and peripheral organ of hemo and lymphopoiesis. It functions as the bursa of Fabricius equivalent in early development, and as it matures, it develops into a peripheral organ of hemo and lymphopoiesis, similar in microscopic structure, and possibly function, to Peyer's patches (Weinstein et al., 1994).
Other authors suggested that mammalian gut-associated lymphoid tissue located at the ileocecal junction, in the place of the highest bacterial concentration, is not the central organ of hemo- and lymphopoiesis for B cells. It may have developed for quick response to an encounter with bacteria in newborns before Peyer's patches and mesenteric lymph nodes take over these functions (Butler & Sinkora, 2013).
Understanding the rabbit appendix structure and functions is necessary for the development of alternative approaches to the prevention and treatment of rabbit diseases, including the improvement of mucosal vaccines and the treatment of inflammatory intestinal diseases (Newberry, 2008). Knowledge about lymphocyte populations in the rabbit appendix remains incomplete (Haines et al., 2016). The detection of common antigens of hematopoietic stem cells between different species does not lose its relevance for translational studies of their biology (Ishibashi et al., 2016). Therefore, the aim of our study was to establish the content and topography of populations of lymphocytes and hematopoietic stem cells in the rabbit cecal appendix.
Materials and Methods
Four clinically healthy 4-months-old male Pannon White rabbits were used in our study. The animals were kept in the vivarium of the National University of Life and Environmental Sciences of Ukraine according to the generally accepted husbandry practices. They were fed ad libitum. Euthanasia, necroscopy of rabbits and collection of tissue samples were carried out according to generally accepted methods on the basis of the laboratory of immunomorphology of the Academician Volodymyr Kasyanenko Department of Animal Anatomy, Histology and Pathomorphology, National University of Life and Environmental Sciences of Ukraine. All interventions and euthanasia of animals were carried out in accordance with norms of bioethics and the requirements of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986) and the resolution of the First National Congress on Bioethics (Kyiv, 2001).
We used monoclonal antibodies CD3, CD10, CD20, and CD34 (DAKO, Denmark) for immunohistochemical studies. Antigenic sites were unmasked using heat induced epitope retrieval with Target Retrieval Solution High pH buffer (DAKO, Denmark) by treatment in Pt Module (Dako, Denmark) for 32 minutes at a temperature of 98-99° Celsius. Visualization of primary antibodies was performed using the DAKO EnVision detection system FLEX+ (DAKO, Denmark). Mayer's hematoxylin was used for a nuclear counterstaining. Preparations were stained for 1-3 minutes followed by placement in Eukitt quick-hardening mounting medium. The obtained tissue sections were examined under an “Olympus” microscope (200x, 400x, 1000x) for establishment of localization and content of lymphoid populations. The number of positive cells was assessed per 10 randomly selected fields of the microscope (at magnification 280x, per conventional unit area). Results are recorded as mean (M) and standard error of mean (m).
Results
CD34 positive cells were detected in the lymphoid tissue of the rabbit cecal appendix. The content of cell populations expressing markers CD3, CD10, CD20, and CD34 differed in nodular and diffuse forms of lymphoid tissue (Table 1).
| Marker | Germinal center of | Dome of lymphoid | Diffuse lymphoid |
|---|---|---|---|
| lymphoid nodule | nodule | tissue | |
| CD3 | 31,67 ± 3,87 | 57,25 ± 2,8 | 147,33 ± 8,91 |
| CD10 | 73,60 ± 1,47 | 90,67 ± 3,29 | 66,75 ± 3,17 |
| CD20 | 124,0 ± 8,59 | 97,0 ± 1,74 | 66,60 ± 9,33 |
| CD34 | 45,25 ± 8,5 | 16,75 ± 1,99 | 2,67 ± 1,36 |
Table 1. The content of lymphoid subpopulations and CD34+ cells in rabbit appendix (cells per conventional unit area), M ± m.
The highest density of T cells (CD3+population of lymphocytes) was found in the diffuse lymphoid tissue between lymphoid nodules, where they represented the largest cell population. A high expression level of this marker characterizes cD3+ cells in this form of lymphoid tissue.
In the nodular form of lymphoid tissue, T cells were located less densely compared to diffuse lymphoid tissue (Figure. 1). Thus, in the domes of lymph nodules their content is 2.6 times, i.e., 61.14% lower than in the diffuse lymphoid tissue. The density of cells expressing the CD3 marker is higher at the periphery of the dome.
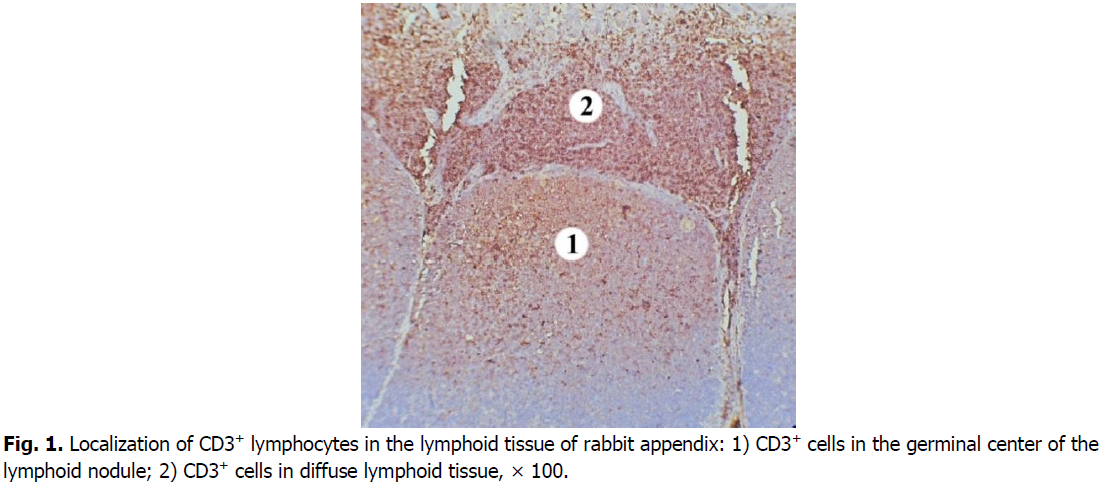
Figure 1: Localization of CD3+ lymphocytes in the lymphoid tissue of rabbit appendix: 1) CD3+ cells in the germinal center of the lymphoid nodule; 2) CD3+ cells in diffuse lymphoid tissue, × 100.
In the germinal centers of secondary lymphoid nodules, CD3+cells are less densely located, there are many lymphoid cells between them that do not express this marker. Mostly membranes of T cells in the germinal centers of lymphoid nodules show the weaker intensity immunostaining with CD3 marker compared to those in diffuse lymphoid tissue (Figure. 2). CD3+cells are mostly in the apical part of the lymphoid nodules. The number of CD3+cells in the germinal centers of the lymphoid nodules is 1.8 times, i.e., 44.68% lower than in the domes of the lymphoid nodules. Compared with the diffuse form of lymphoid tissue, the number of cells that express the CD3 marker in the germinal centers of lymphoid nodules is 4.7 times, i.e., 78.5% lower.
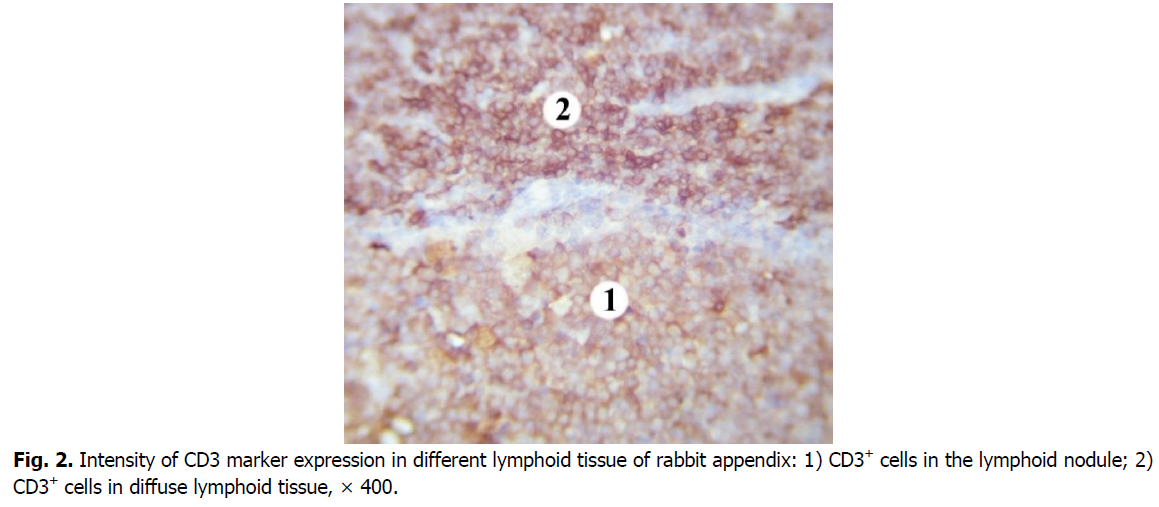
Figure 2: Intensity of CD3 marker expression in different lymphoid tissue of rabbit appendix: 1) CD3+ cells in the lymphoid nodule; 2) CD3+ cells in diffuse lymphoid tissue, × 400.
Clusters of CD3+cells as chains are also in the walls of the crypts. Single T cells infiltrate the follicle-associated epithelium over the lymphoid nodules and the tela submucosa between the lymphoid nodules.
We registered the largest number of B cells in the nodular form of the lymphoid tissue of the rabbit cecal appendix in the germinal centers of the lymphoid nodules. Their number is lower in the basal part of the lymphoid nodules. In this region, cells expressing the CD20 marker are less densely located and are predominantly localized in its center.
B cells have a low density of distribution in the domes of lymphoid nodules. Compared to the germinal centers of the lymphoid nodules, the number of CD20+ cells in the domes of the lymphoid nodules is 1.3 times and 21.77% lower. The diffuse form of lymphoid tissue has the lowest density of B cells (Figure. 3). Their number is 1.5 times, i.e. 31.34% lower than in the domes of lymphoid nodules, and 1.9 times or 46.29% lower compared with the germinal centers. B cells are also gathered in chain-like clusters or located as single cells in the walls of the crypts.
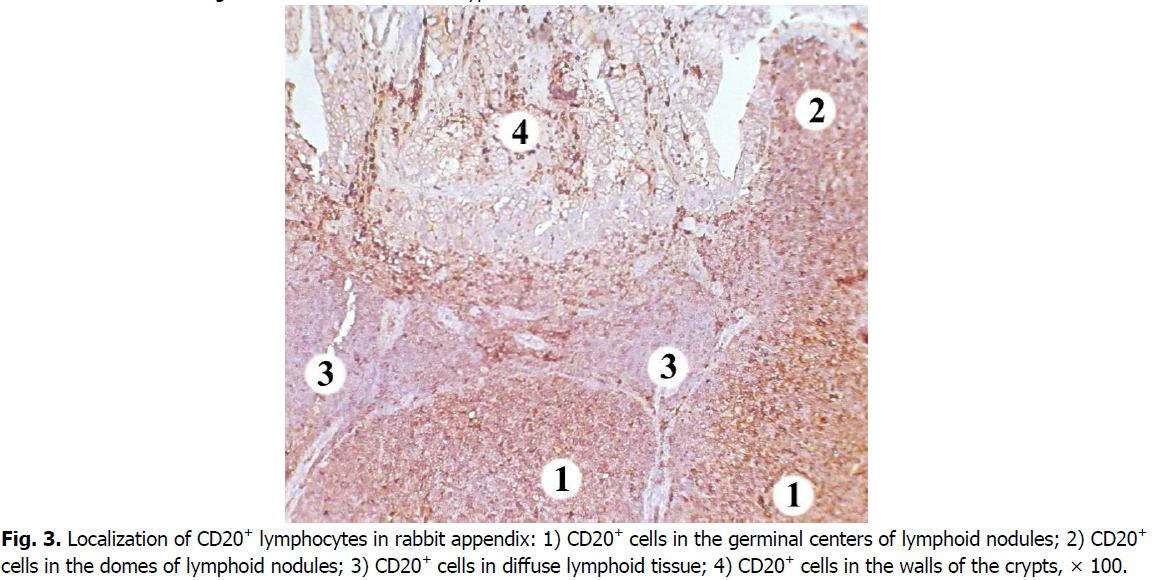
Figure 3: Localization of CD20+ lymphocytes in rabbit appendix: 1) CD20+ cells in the germinal centers of lymphoid nodules; 2) CD20+ cells in the domes of lymphoid nodules; 3) CD20+ cells in diffuse lymphoid tissue; 4) CD20+ cells in the walls of the crypts, × 100.
Cells expressing the CD10 marker are predominantly concentrated in the nodular form of the lymphoid tissue of the rabbit cecal appendix. The highest density of B and T cell precursors is characteristic for the domes and germinal centers of lymphoid nodules (here their number is 18.83% lower). In the basal part of the lymphoid nodules and the mantle zone, density of CD10+ cells is lower, as well as the level of membrane expression of this marker.
A smaller number of CD10+ cells is found in the diffuse form of lymphoid tissue: it is 9.31% lower than in the germinal centers of lymphoid nodules; and 1.4 times, i.e., 26.38% lower compared to the domes of lymphoid nodules. Clusters of CD10+cells are also located in the walls of the crypts (Figure. 4).
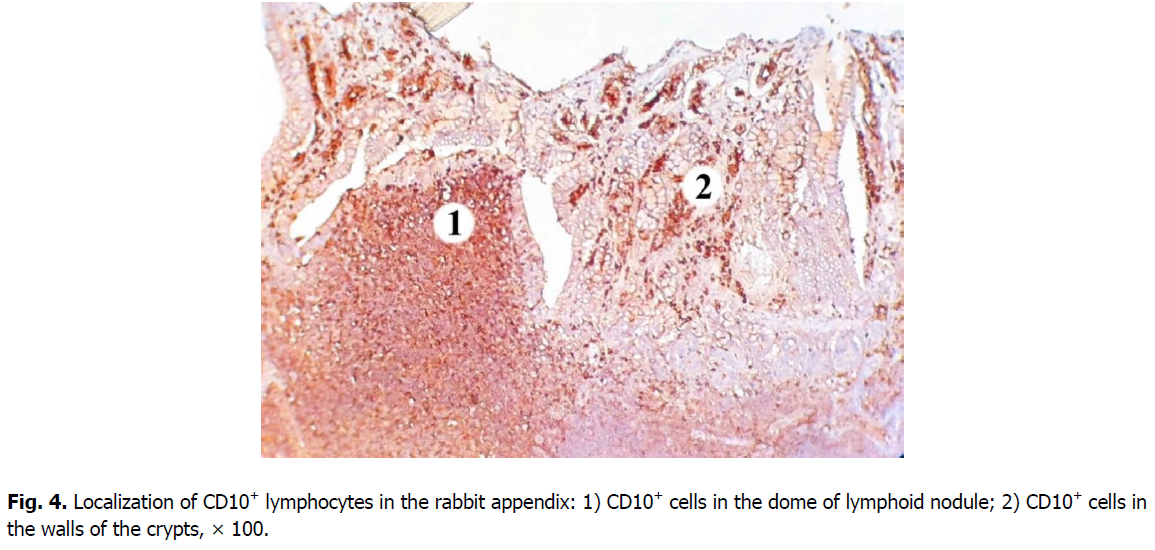
Figure 4: Localization of CD10+ lymphocytes in the rabbit appendix: 1) CD10+ cells in the dome of lymphoid nodule; 2) CD10+ cells in the walls of the crypts, × 100.
The majority of cells expressing CD34 marker is concentrated in the central and apical parts of germinal centers of the nodules (Figure. 5). The membranous staining pattern is characteristic for CD34+cells and therefore they have brown rims (Figure. 6). CD34+ cells were found in each lymphoid nodule. On the periphery of the lymphoid nodules and in their basal part, there are only single cells expressing this marker. The level of membrane expression of CD34 cells in lymphoid nodules varies from weak (mainly in the periphery) to medium (in the center).
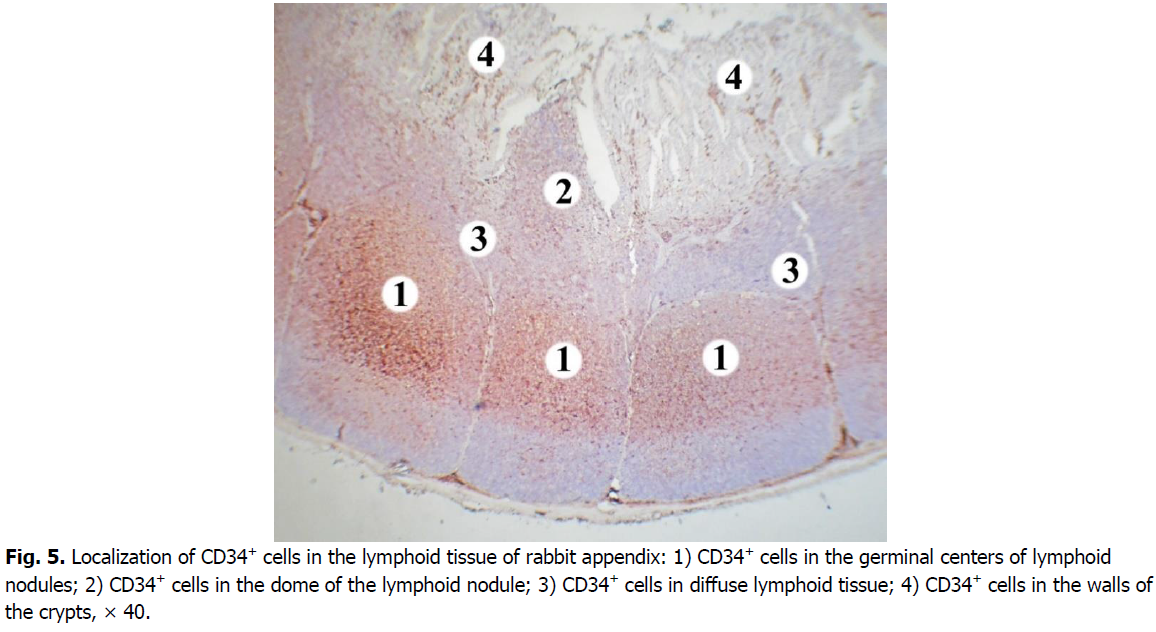
Figure 5: Localization of CD34+ cells in the lymphoid tissue of rabbit appendix: 1) CD34+ cells in the germinal centers of lymphoid nodules; 2) CD34+ cells in the dome of the lymphoid nodule; 3) CD34+ cells in diffuse lymphoid tissue; 4) CD34+ cells in the walls of the crypts, × 40.
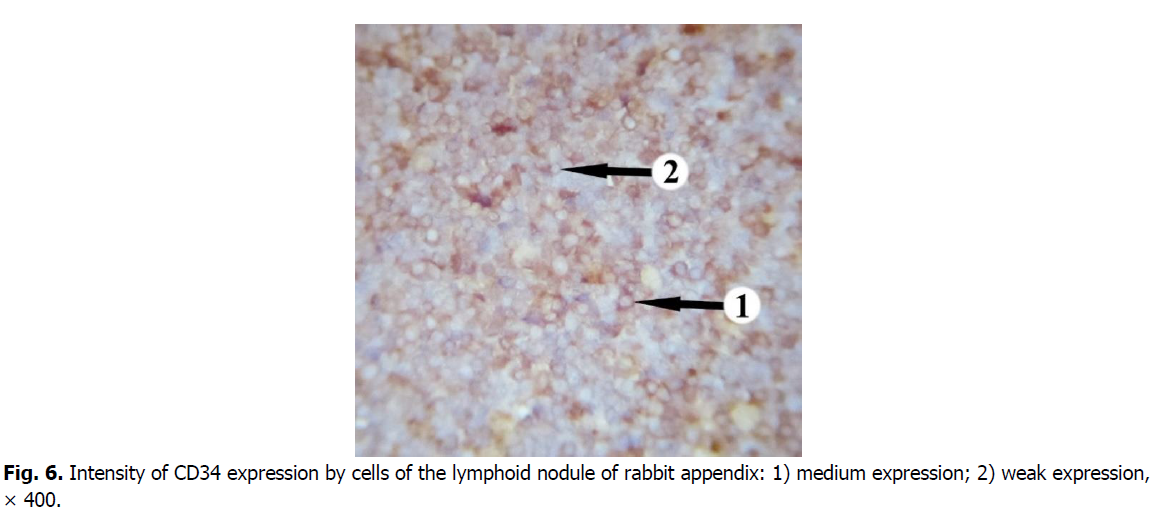
Figure 6: Intensity of CD34 expression by cells of the lymphoid nodule of rabbit appendix: 1) medium expression; 2) weak expression, × 400.
In the domes of lymphoid nodules, the density of CD34+ cells is lower compared to the germinal centers. Their number is 2.7 times, i.e. 62.98% lower. Only a few CD34+ cells were found in the diffuse form of lymphoid tissue. The number of cells expressing CD34 in diffuse lymphoid tissue is 16.9 times, i.e. 94.1% lower compared to the germinal centers of lymphoid nodules. The difference in the number of CD34+ cells in the domes of lymphoid nodules is somewhat less pronounced: in the diffuse lymphoid tissue their number is 6.3 times, i.e. 84.06% lower.
In the walls of the crypts the density of cells expressing CD34 is low, they contain only single CD34+ cells.
Discussion
Albeit the antibodies used in this study were developed against the corresponding antigens in humans, we know that some antigens, such as CD3, are common to many species. It was proven that human markers can be quite effective in mouse studies (Rehg et al., 2012). Other researchers have reported that rabbit B cells express markers CD10 and CD20, as well as in humans (Yeramilli, 2010). Also, studies have shown the efficiency of human monoclonal antibodies for detection of the rabbit CD34+ hematopoietic stem cells (Vašícek et al., 2018). Confirmation of a positive specific reaction to the antibodies we used was their characteristic type of expression (membranous).
CD3 is a complex structure that consists of several protein chains and associates with the T cell receptor (antigen-specific receptor of T cells). It is usually located on the surface membrane of mature T cells and in the cytoplasm of immature T-lymphoblasts, and sometimes NK cells. In addition, plasma cells and macrophages can also have non-specific cytoplasmic staining (Naeim et al., 2018). The CD10 marker is also known as neutral endopeptidase or common acute lymphoblastic leukemia antigen (CALLA). It is expressed on early lymphoid progenitors, disappears during their maturation, and can reappear on activated B lymphocytes. This molecule can also be present on fibroblasts, some epithelial cells, granulocytes and follicular dendritic cells (Delves et al., 2011) (Naeim et al., 2018).
CD20 is a common marker for identification of B cells. It is a surface molecule embedded into membrane, which plays a role in the B cells development and differentiation into plasma cells. This marker appears after CD10 expression in the ontogeny of B lymphocytes. CD20 is expressed throughout differentiation of B cells before their terminal differentiation to plasma cells. It can also be expressed by follicular dendritic cells (Naeim et al., 2018) (Delves et al., 2011) (Murphy & Weaver, 2017).
CD34 is a surface glycoprotein expressed on hematopoietic stem cells, progenitor cells and endothelial cells of small vessels. Some cells in nervous tissue and embryonic fibroblasts are CD34-positive. Pro-B cells and TdT+ subset of B-cell precursors (pre-B cells in which DNA polymerase terminal deoxynucleotidyl transferase is present) also express this marker. Studies suggest that CD34 is involved in regulation of migration, differentiation and proliferation of blood cells, possibly as a cell adhesion molecule (Naeim et al., 2018) (Krause et al., 1996) (Sidney et al., 2014) (Lin et al., 1995) (Sanz et al., 2010) (Weiss & Wardrop, 2010).
Hematopoietic stem cells are a small group of cells that are maintained throughout life (capable of self-renewal). They give rise to hematopoietic progenitor cells that further differentiate into many mature blood cells. Among markers of hematopoietic stem and progenitor cells, CD34 is well known for its unique expression on them (AbuSamra et al., 2017).
Studies indicate that IgM+ B cells with an undiversified repertoire from the bone marrow are transported with the peripheral blood, and colonize the appendix in neonatal rabbits (Pospisil et al., 2006) (R. K. Sinha et al., 2006) (R. Sinha, 2004). Under the influence of intestinal commensal bacteria, they experience further primary diversification by 3 weeks of age through gene conversion and somatic hypermutation, and generate a wide repertoire of antibodies (Girard-Madoux et al., 2018) (R. Sinha, 2004). In the appendix B-cell multiplication, diversification of immunoglobulins, clonal expansion and selection take place. Those that survive the selection process exit the appendix and form the preimmune repertoire in the periphery (R. Sinha, 2004) (R. K. Sinha et al., 2006).
In rabbits, the percentage of B lymphocyte precursors in the bone marrow reaches its maximum of 10% at birth, and at 3 weeks of age it decreases to 1-3%. This indicates that B lymphopoiesis decreases with age, and is practically absent in adult rabbits. Peripheral B cells of adult rabbits might be long lived or self-renewing (Hanson & Lanning, 2008) (Dennis Lanning et al., 2000) (Pastoret et al., 1998).
Although most intestinal lymphocytes are believed to have homed from the periphery, it is possible that some of them have differentiated from intestinal hematopoietic stem cell populations (Lynch et al., 2006).
Both lymphoid progenitors and hematopoietic stem cells have been described in the murine intestine. There is evidence that differentiation of T cells also takes place in the human neonatal intestinal tract, and that the adult human intestine may support lymphopoiesis. Over 5% of leukocytes isolated from the human intestine also express CD34, which is significantly higher than in peripheral blood or bone marrow (Lynch et al., 2006). Among the ileal lamina propria lymphocytes, the percentage of hematopoietic stem cells is significantly higher than among intraepithelial lymphocytes (Fu et al., 2017) (Fu et al., 2019). The phenotype of intestinal hematopoietic stem cells differs from bone marrow or circulating hematopoietic stem cells and corresponds to their functions in local lymphoid development (Urbiztondo, 2010) (Lynch et al., 2006).
Recent studies have shown that the animal and human appendix is one site where hematopoietic progenitor cells, which are located in intraepithelial areas and lamina propria, are most abundant (De Coppi et al., 2006).
In the intestine, only a single layer of epithelial cells separates the gut lumen from the internal environment of an organism. From an evolutionary point of view, the intestinal tract of higher vertebrates may keep some self-supporting system to ensure internal integrity (Saito et al., 1998).
The results of our study show that the rabbit vermiform appendix contains CD34+ cells, which may indicate the presence of hematopoietic stem cells in it. They are mostly located in the germinal centers of secondary lymphoid nodules. In the domes of lymphoid nodules and diffuse lymphoid tissue their number is 2.7 and 16.9 times lower, respectively. Single CD34+ cells were found in the walls of the crypts. Among the lymphoid subpopulations in the nodular form of the rabbit appendix lymphoid tissue, CD20+ cells (B cells) predominate. It also contains many CD10+ cells (precursors of T cells and B cells), which are mainly concentrated in the germinal centers of lymphoid nodules. In the diffuse form of lymphoid tissue, CD3+cells (T cells) represent the vast majority of cells.
References
AbuSamra, D.B., Aleisa, F.A., Al-Amoodi, A.S., Jalal Ahmed, H.M., Chin, C.J., Abuelela, A.F., Merzaban, J.S. (2017). Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Advances, 1:2799-2816.
Aminova, G.G. (2019). Structure and Cytoarchitectonics of Lymphoid Tissue in Appendi?es of Elderly and Senile People. Advances in Gerontology, 9:109-114.
Archer, O.K., Sutherland, D.E., Good, R.A. (1963). Appendix of the rabbit: a homologue of the bursa in the chicken? Nature, 200:337-339.
Butler, J.E., Sinkora, M. (2013). The enigma of the lower gut- associated lymphoid tissue (GALT). Journal of Leukocyte Biology, 94:259-270.
Dasso, J.F., Obiakor, H., Bach, H., Anderson, A.O., Mage, R.G. (2000). A morphological and immunohistological study of the human and rabbit appendix for comparison with the avian bursa. Developmental & Comparative Immunology, 24:797-814.
Davies, R.R., Davies, J.A.R. (2003). Rabbit gastrointestinal physiology. Veterinary Clinics: Exotic Animal Practice, 6:139-153.
De Coppi, P., Pozzobon, M., Piccoli, M., Gazzola, M.V., Boldrin, L., Slanzi, E., Gamba, P. (2006). Isolation of mesenchymal stem cells from human vermiform appendix. Journal of Surgical Research, 135:85-91.
Delves, P.J., Martin, S.J., Burton, D.R., Roitt, I.M. (2017). Roitt's essential immunology. John Wiley & Sons.
Fu, J., Zuber, J., Martinez, M., Shonts, B., Obradovic, A., Wang, H., Sykes, M. (2019). Human intestinal allografts contain functional hematopoietic stem and progenitor cells that are maintained by a circulating pool. Cell Stem Cell, 24:227-239.
Fu, J., Zuber, J., Shonts, B., Wang, H., Lau, S.P., Savage, T., Sykes, M. (2017). Phenotype and Function of Human Gut Hematopoietic Stem Cells and Progenitors. Transplantation, 101:S5-S6.
Girard-Madoux, M.J., de Agüero, M.G., Ganal-Vonarburg, S.C., Mooser, C., Belz, G.T., Macpherson, A.J., Vivier, E. (2018). The immunological functions of the appendix: an example of redundancy?. In Seminars in Immunology, 36:31-44. Academic Press. Haines, R.A., Urbiztondo, R.A., Haynes, R.A., Simpson, E., Niewiesk, S., Lairmore, M.D. (2016). Characterization of new zealand white rabbit gut-associated lymphoid tissues and use as viral oncology animal model. ILAR Journal, 57:34-43.
Hanson, N.B., Lanning, D.K. (2008). Microbial induction of B and T cell areas in rabbit appendix. Developmental & Comparative Immunology, 32:980-991.
Hulls, C. (2015). Spatiotemporal mapping of the motility of the ex vivo rabbit caecum: a thesis presented in partial fulfilment of the requirements for the degree of Masters of Physiology in Digestive Biomechanics (Physical Process of Digestion) at Massey University, Turitea, New Zealand (Doctoral dissertation, Massey University).
Tomohiko. I. (2016). ESAM is a novel human hematopoietic stem cell marker associated with a subset of human leukemias.
Kooij, I.A., Sahami, S., Meijer, S.L., Buskens, C.J., Te Velde, A.A. (2016). The immunology of the vermiform appendix: a review of the literature. Clinical & Experimental Immunology, 186:1-9.
Krause, D.S., Fackler, M.J., Civin, C.I., May, W.S. (1996). CD34: structure, biology, and clinical utility.
Kuper, C.F., Wijnands, M.V., Zander, S.A. (2017). Mucosa-associated lymphoid tissues. In Immunopathology in toxicology and drug development. Humana Press, Cham, pp: 81-121.
Lanning, D., Sethupathi, P., Rhee, K.J., Zhai, S.K., Knight, K.L. (2000). Intestinal microflora and diversification of the rabbit antibody repertoire. The Journal of Immunology, 165:2012-2019.
Lanning, D., Zhu, X., Zhai, S.K., Knight, K.L. (2000). Development of the antibody repertoire in rabbit: gut- associated lymphoid tissue, microbes, and selection. Immunological reviews, 175:214-228.
Lin, G., Finger, E., Gutierrez- Ramos, J.C. (1995). Expression of CD34 in endothelial cells, hematopoietic progenitors and nervous cells in fetal and adult mouse tissues. European journal of immunology, 25:1508-1516.
Lynch, L., O’Donoghue, D., Dean, J., O’Sullivan, J., O’Farrelly, C., Golden-Mason, L. (2006). Detection and characterization of hemopoietic stem cells in the adult human small intestine. The Journal of Immunology, 176:5199-5204.
Mage, R.G., Pinheiro, A., de Matos, A.L., Esteves, P.J. (2016). The immune system of lagomorphs.
Murphy K., Weaver. C. (2017). Janeway’s Immunobiology. New York, NY: Garland Science, Taylor & Francis Group, LLC, p: 904. Naeim, F., Nagesh Rao, P., Song, S.X., Grody, W.W. (2013). Principles of immunophenotyping. Atlas of Hematopathology, 1034:25- 46.
Newberry, R.D. (2008). Intestinal lymphoid tissues: is variety an asset or a liability?. Current Opinion in Gastroenterology, 24:121- 128.
Tully, T.N. (2005). Clinical Anatomy and Physiology of Exotic Species: Structure and function of mammals, birds, reptiles and amphibians. In Seminars in Avian and Exotic Pet Medicine, p: 269.
Parker, G.A. (2017). Development of immune system organs. In Immunopathology in Toxicology and Drug Development, pp:245- 294.
Pastoret, P.P., Griebel, P., Bazin, H., Govaerts, A. (Eds.). (1998). Handbook of vertebrate immunology. Academic Press.
Pospisil, R., Alexander, C.B., Obiakor, H., Sinha, R.K., Mage, R.G. (2006). CD5+ B cells are preferentially expanded in rabbit appendix: the role of CD5 in B cell development and selection. Developmental & Comparative Immunology, 30:711-722.
Rehg, J.E., Bush, D., Ward, J.M. (2012). The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicologic Pathology, 40:345- 374.
Saito, H., Kanamori, Y., Takemori, T., Nariuchi, H., Kubota, E., Takahashi-Iwanaga, H., Ishikawa, H. (1998). Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science, 280:275-278.
Sanz, E., Muñoz-A, N., Monserrat, J., Van-Den-Rym, A., Escoll, P., Ranz, I., de-la-Hera, A. (2010). Ordering human CD34+ CD10- CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proceedings of the National Academy of Sciences, 107:5925-5930.
Sidney, L.E., Branch, M.J., Dunphy, S.E., Dua, H.S., Hopkinson, A. (2014). Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells, 32:1380-1389.
Sinha, R.K., Mage, R.G. (2004). Developing neonatal rabbit appendix, a primary lymphoid organ, is seeded by immature blood- borne B cells: evidence for roles for CD62L/PNAd, CCR7/CCL21, a4ß1 and LFA-1. Developmental & Comparative Immunology, 28:829-841.
Sinha, R.K., Alexander, C., Mage, R.G. (2006). Regulated expression of peripheral node addressin-positive high endothelial venules controls seeding of B lymphocytes into developing neonatal rabbit appendix. Veterinary Immunology and Immunopathology, 110:97-108.
Smith, H.F., Parker, W., Kotzé, S.H., Laurin, M. (2013). Multiple independent appearances of the cecal appendix in mammalian evolution and an investigation of related ecological and anatomical factors. Comptes Rendus Palevol, 12:339-354.
STAN, F. (2014). Anatomical Particularities of the Cecum in Rabbits and Chinchillas. Bulletin of the University of Agricultural Sciences & Veterinary Medicine Cluj-Napoca. Veterinary Medicine, 71.
Urbiztondo, R.A. (2010). Studies of gut-associated lymphoid tissues and other secondary lymphoid tissues in 12 week old New Zealand White specific pathogen free rabbits (Doctoral dissertation, The Ohio State University).
Varga, M., Harcourt-Brown, F. (2014). Textbook of rabbit medicine. 2nd ed. Edinburgh; New York: Elsevier, p:494.
Vašícek, J., Shehata, M., Schnabl, S., Hilgarth, M., Hubmann, R., Jäger, U., Chrenek, P. (2018). Critical assessment of the efficiency of CD34 and CD133 antibodies for enrichment of rabbit hematopoietic stem cells. Biotechnology Progress, 34:1278-1289.
Weinstein, P.D., Mage, R.G., Anderson, A.O. (1994). The appendix functions as a mammalian bursal equivalent in the developing rabbit. In In Vivo Immunology, pp:249-253.
Weiss, D.J., Wardrop, K.J. (Eds.). (2011). Schalm's veterinary hematology. John Wiley & Sons.
Yeramilli, V.A. (2010). B lymphocyte development in GALT. Loyola University Chicago.
Author Info
Khomych, V.T., Fedorenko, O.V.*, Mazurkevych, T.A. and Dyshlyuk, N.VCitation: Khomych, V.T., Fedorenko, O.V., Mazurkevych, T.A., Dyshlyuk, N.V. (2021). Immunohistochemical characterisation of lymphoid subpopulations and CD34+ cells in the lymphoid tissue of rabbit appendix. Ukrainian Journal of Ecology, 11 (5), 1-8.
Received: 04-Jun-2021 Accepted: 14-Jun-2021 Published: 29-Jul-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
