Research - (2022) Volume 12, Issue 5
Identification of novel sources of resistance for fusarium head blight among ethiopian bread wheat germplasm
G.M. Abebele*Abstract
Wheat is one of the world's leading crops and takes a significant role for food security around the globe. The production of wheat becomes large over time both in area coverage and volume of grain production. Despite the expansion, production and productivity of wheat is constrain by several fungal pathogens of which FHB is one that caused huge losses in both quantity and quality. This study was executed with the intention to ascertain promising sources of fusarium headlight resistance among Ethiopian bread wheat breeding pipelines for durable resistance breeding. A total of 578 (288 PVT and 290 NVT) advanced bread wheat pipelines, along with local susceptible check cultivars Alidoro and Wane were field tested in partially replicated augmented design at Kulumsa agricultural research center, hot-spot for fusarium headblight for their field response. Diseases severity data was assessed based on a standard five digit rating scale and put in to different classes. Out of 288 PVT lines 7.3%, 35%, 41.6%, 12.8% and 3.1%; and out of 290 NVT lines 1.7%, 51.1%, 32.4%, 14.1% and 0.7% showed immune, resistant, moderately resistant, moderately susceptible and susceptible response respectively. However, none of the lines had very susceptible infection types, >75% severity. On the other hand, susceptible check varieties scored 5(>75%) diseases severity indicating that season and location were ideal to screen genotypes. The result confirmed that considerable amount of test genotypes namely; EBW193155, EBW202086, EBW202084, EBW202116, EBW193169, EBW202005, EBW192386, EBW202025, EBW192907, EBW192065, EBW193404, EBW193105 and EBW202057 from Pre-variety trials as well as EBW150113, EBW193004, EBW193027, EBW193045, EBW150046, EBW150047 and EBW150048 from national variety trials (NVT) showed immune to resistant infection type along with promising agronomic performance thus, could be used for durable FHB resistance breeding in wheat.
Keywords
Wheat, Fusarium head blight, Breeding pipelines, NVT, PVT.
Introduction
Wheat is the second most cultivated cereal crops in the globe following to rice (Oryza sativa L.) with a production of 881.16 million tons produced on 244.46 million hectares of land with an average yield of 3.65 tons per hectare (FAO, 2018). The economic significance of wheat and its role to the diets of humans and livestock cannot be doubtful. Its demand is increasing in countries undergoing urbanization and industrialization (Peter R., 2015). At present, available data showed an average annual global production of about at 769.6 million tonnes (https://www.fao.org/worldfoodsituation/csdb/en/).
In Ethiopia, wheat is the fourth most widely grown crop (with coverage of 1.70 million hectares) next to teff (Eragrostis tef (Zucc.) Trotter), maize or corn (Zea mays subsp. mays L.) and sorghum (Sorghum bicolor L.) and ranks third in terms of the gross production (4.54 million tons) after maize and teff (CSA, 2017). In the last two decades, many government programs and initiatives run to drive agricultural growth and food security in Ethiopia, resulting in a significant increase in wheat production from 1.10 million tons in 1995/96 (Bergh et al., 2012) to 5.8 million tons in 2021 (CSA, 2021). Wheat obtained a great attention because it is one of the poverty alleviation crops of the country (Demeke, 1999). In line with the government's plan and need, there are a lot of works made by the regional, national and international organizations giving due attention to variety development, demonstration, dissemination of wheat technologies and more recently encouraging cluster farming to aid wheat mechanization in Ethiopia (Gebreab, 2006).
Despite the rapid increase of wheat in area coverage and productivity, there are many production restraints leading the wheat productivity in Ethiopia to 2.68 t ha-1 (CSA, 2017) below the global yield of 3.65 t ha-1 (FAO, 2018). In general, this low yield of wheat is a result of various biotic factors like diseases, insects and weeds; abiotic factors including lack of moisture due to uneven distribution of the rainfall, field size, flooding, hail, soil fertility and acidity problems and continuous farming; technical and socio-economic factors like less adoption of new technology, limited access to credit; and climatic factor like temperature rise beyond optimum for wheat (Barron et al., 2003; Liu et al., 2008; Hailu et al., 2011; Mann and Warner, 2015). Among the pests, fungal diseases like Puccinia spp., Septoria spp. and Fusarium spp. are the main constraints to wheat production in East Africa including Ethiopia. Nowadays, Fusarium head blight (FHB) also called scabs or tombstone of wheat obtain the biggest concern (Tesfaye and Pim, 2016).
Fusarium head blight is one of the utmost destructive diseases of bread wheat (Triticum aestivum L.) and durum wheat (Triticum durum Desf.) worldwide, which leads to significant losses in grain yield and quality. The diseases are primarily caused by members of the fusrium graminearum but also by F. culmurum and F. avenaceum in cooler regions (Parry et al. 1995). Most importantly these fungi are able to produce mycotoxins that accumulate in the grains and constitute a serious threat to food safety (Pestka, 2010).
During the past three decades, FHB has emerged as a major threat to wheat at global levels with an increasing trend of epidemics (McMullen et al., 2012). Several outbreaks with severe on wheat have been experienced since 1990 in different parts of the world. For instance, in USA, economic losses of $ 2.7 billion were estimated on wheat due to FHB epidemics during 1998 to 2000s in North Dakota and Minnesota (Nganje et al., 2004a).
In sub-Saharan Africa (SSA), there is lack of information regarding the FHB epidemics and economic losses on wheat because of the underdeveloped research on the disease (Dweba et al., 2017). Particularly in Ethiopia, there is little information on FHB of wheat that reported the disease as one of the major wheat diseases at high altitude areas (Bekele, 1985) and the 1988 cropping season was one of the scabby season with an incidence of 85% and severity of 5-80% (Bekele, 1990). Besides, the disease was reported to cause yield losses of 60% and above under experimental conditions in 1989 cropping season of Ethiopia (Snijders, 1989). Moreover, the Fusarium spp. associated with FHB of wheat across Arsi, Bale, Gojam, Gonder, Shoa and Wollo areas were identified into 17 Fusarium spp. during 1987 and 13 Fusarium spp. during 1989 (Bekele, 1990), but their pathogenicity was not verified. Almost two decades later, a new novel specie named as F. aethiopicum was phylogenetically identified among the 31 isolates of F. graminearum species complex collected from Gugsa-Womberma and Bure in Amhara region and Arsi-Robe in Oromia region (O’Donnell et al., 2008) this might indicate the existence of species diversity in Ethiopia. Meanwhile, less concern was given to FHB of wheat in Ethiopia even though it is a potent disease and also there is a diversity of Fusarium pathogens in the country.
The relevance of the selection of genotypes resistant to FHB in wheat is based on the rationale that host resistance is considered the most appropriate means of controlling the disease (Pereyra and Lori 2013). The incidence and severity of a disease may help to characterize its phenotypes (Engle et al. 2003,). Thus, the current study was conducted to evaluate wheat germplasm against FHB under natural infestation conditions.
Materials and Methods
Descriptions of the study area
The trial was executed at Kulumsa agricultural research center, wheat research regional center of excellence in 2021 cropping season. Geographically, the center is found at 080 01'10''N, 390 09'11''E and at 2200 meters above sea level (m.a.s.l). It receives mean annual rainfall of 820 mm representing highland and high rainfall agro ecology. The monthly mean minimum and maximum temperature is 10.5 and 22.8°C respectively. The site’s dominant soil type is loam which is fertile.
Plant materials
The examined spring wheat genotypes were obtained from Ethiopian national bread wheat research center of excellence; Kulmsa agricultural research center which were prior introduced from CIMMYT and ICARDA. The genotypes were evaluated for yield and rust diseases resistance and were found at advanced (PVT and NVT) breeding stages. The experimental set also included 10 commercial bread wheat varieties used in Ethiopia.
Field experiment and Design
Field design
In this study 577 bread wheat genotypes and 10 commercial varieties germplasms, introduced from CIMMYT, ICARDA and developed locally by crossing were screened against rusts and fusarium head blight in naturally infested fields. The experiment was arranged in partially replicated design in two sets each set consisting of 288 genotypes. Entries were planted in 1m length with 0.6m space between rows length with two rows and 10 blocks. All agronomic practices were applied uniformly for all plots.
Disease evaluation
For scoring we assumed an average head-size of 24-28 spikelets per spike as the basis for estimating FHB severity; e.g. an average of one infected spikelet per spike was rated as 5% FHB severity. In each plot the percentage of visually infected spikelets was estimated according to a linear scale 0 to 100% infected spikelets on a whole plot basis. Disease severity ranges were scored according to the Japanese and Brazilian scale (Kohli, 1989) based on spike severity where: 0, immune; 1, resistant (1-5% severity); 2, moderately resistant (5-25% severity); 3, moderately susceptible (25-50% severity), 4, susceptible (50-75% severity); 5, very susceptible (>75% severity).
Results and Discussion
Over the past long years, phenotypic assortment was used as the only option in resistance breeding to develop improved FHB resistant wheat genotypes. Phenotypic selection is still superior over genomic selection when time and cost are not deliberated and attaining high-quality phenotypic data will definitely stay essential for further progress in resistance improvement.
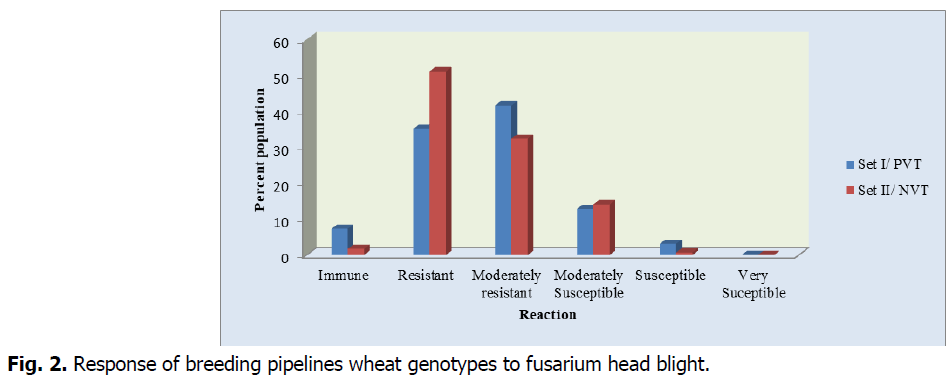
Disease severity data was recorded based on a 0 to 5 rating scale. Although the diseases pressure under the growing season was not as such high, the result revealed there was wide variation in severity among evaluated genotypes ranging from immune to susceptible. Out of 288 PVT lines 7.3%, 35%, 41.6%, 12.8% and 3.1% of the screened materials showed immune, resistant, moderately resistant, moderately susceptible and susceptible response. However, none of the lines had very susceptible infection types, >75% severity revealing that diseases pressure was not too epidemic.
Likewise, out of 290 NVT breeding pipelines 1.7%, 51.1%, 32.4%, 14.1% and 0.7% of the screened materials showed immune, resistant, moderately resistant, moderately susceptible and susceptible response. However, none of the lines had very susceptible infection types, >75% severity indicated that diseases stress was not too epiphytotic. The result presented in Fig. 1 and 2.
Fig 1: Status of FHB on a selected plot under field condition.
Fig 2: Response of breeding pipelines wheat genotypes to fusarium head blight.
Host genetic resistance is the utmost economic and effective means of reducing yield losses and quality deterioration caused fusaria spp. (Steiner et al., 2017). In the current work, majority of the test lines of both PVT and NVT exhibited beyond moderately resistant infection types. However, this result might not be due to the presence of reasonable number of resistant lines in the population but, probably it happened because of the amount of inoculum in the soil in the form of debris or planted seeds were free of fusaria which might have created sufficient infection; or the weather condition was not conducive to favor the occurrence of the diseases. This predicted declaration could be supported by Schmale et al. 2003, which states warm, wet weather with high relative humidity during flowering (anthesis) will favor the development of fusarium head blight (scab).
Similar studies have been practiced since the early 1990s, to search, develop and use of resistant wheat varieties for the control of FHB in USA. According to UGA, 2014, it is impossible to get completely resistant wheat lines among the thousands of evaluated genotypes. So scientists recommend the producers to use those lines having reduced fungal growth and low levels of seed contamination.
The resistant wheat lines that exhibited immune and resistant infection types can be incorporated into wheat as new sources of resistance to escape heavy yield losses associated with the diseases. These results were supported by the findings of other researchers (Hussain et al., 2011; Kolmer et al., 2007; Stepien et al., 2003). Developing disease resistance genotypes is an incessant process and plant breeders need to incorporate new effective genes to their breeding materials. Resistance expression rest on on the host-parasite interaction, environmental conditions, plant growth stage and the interaction between resistance genes in wheat genome (Kolmer, 1996). New sources of resistance could be incorporated into wheat to diverse the existing gene pool for FHB resistance (UGA, 2014) (Tables 1 and 2).
| Genotype | Sev. | Genotype | Sev. | Genotype | Sev. | Genotype | Sev. | Genotype | Sev. | Genotype | Sev. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EBW150020 | 0 | EBW150049 | 1 | EBW192406 | 1 | EBW192909 | 1 | EBW193154 | 1 | EBW202036 | 1 |
| EBW150075 | 0 | EBW150051 | 1 | EBW192443 | 1 | EBW192920 | 1 | EBW193155 | 1 | EBW202047 | 1 |
| EBW150076 | 0 | EBW150060 | 1 | EBW192515 | 1 | EBW192921 | 1 | EBW193165 | 1 | EBW202049 | 1 |
| EBW150077 | 0 | EBW150062 | 1 | EBW192783 | 1 | EBW192928 | 1 | EBW193166 | 1 | EBW202057 | 1 |
| EBW150078 | 0 | EBW150074 | 1 | EBW192789 | 1 | EBW192945 | 1 | EBW193167 | 1 | EBW202061 | 1 |
| EBW192386 | 0 | EBW150079 | 1 | EBW192793 | 1 | EBW192973 | 1 | EBW193172 | 1 | EBW202063 | 1 |
| EBW192762 | 0 | EBW150101 | 1 | EBW192800 | 1 | EBW193076 | 1 | EBW193174 | 1 | EBW202067 | 1 |
| EBW193115 | 0 | EBW150103 | 1 | EBW192802 | 1 | EBW193080 | 1 | EBW193183 | 1 | EBW202071 | 1 |
| EBW193169 | 0 | EBW150142 | 1 | EBW192803 | 1 | EBW193084 | 1 | EBW193403 | 1 | EBW202074 | 1 |
| EBW193404 | 0 | EBW150152 | 1 | EBW192807 | 1 | EBW193095 | 1 | EBW194076 | 1 | EBW202079 | 1 |
| EBW202028 | 0 | EBW192005 | 1 | EBW192810 | 1 | EBW193097 | 1 | EBW194107 | 1 | EBW202081 | 1 |
| EBW202075 | 0 | EBW192021 | 1 | EBW192811 | 1 | EBW193100 | 1 | EBW194152 | 1 | EBW202083 | 1 |
| EBW202086 | 0 | EBW192065 | 1 | EBW192814 | 1 | EBW193103 | 1 | EBW194158 | 1 | EBW202084 | 1 |
| EBW202087 | 0 | EBW192151 | 1 | EBW192815 | 1 | EBW193106 | 1 | EBW194194 | 1 | EBW202099 | 1 |
| EBW202093 | 0 | EBW192170 | 1 | EBW192817 | 1 | EBW193113 | 1 | EBW202006 | 1 | EBW202104 | 1 |
| EBW202109 | 0 | EBW192211 | 1 | EBW192818 | 1 | EBW193114 | 1 | EBW202014 | 1 | EBW202105 | 1 |
| EBW202116 | 0 | EBW192253 | 1 | EBW192821 | 1 | EBW193124 | 1 | EBW202018 | 1 | EBW202115 | 1 |
| EBW202117 | 0 | EBW192331 | 1 | EBW192823 | 1 | EBW193126 | 1 | EBW202025 | 1 | EBW202155 | 1 |
| EBW202170 | 0 | EBW192350 | 1 | EBW192828 | 1 | EBW193127 | 1 | EBW202027 | 1 | EBW202433 | 1 |
| EBW192364 | 1 | EBW192856 | 1 | EBW193137 | 1 | EBW202035 | 1 | EBW202441 | 1 |
Table 1. Reaction of promising PVT bread wheat lines selected based on lower FHB good agronomic performance.
| Genotype | Severity | Genotype | Severity | Genotype | Severity | Genotype | Severity | Genotype | Severity |
|---|---|---|---|---|---|---|---|---|---|
| EBW150113 | 0 | EBW192154 | 1 | EBW192757 | 1 | EBW192915 | 1 | EBW193172 | 1 |
| EBW193004 | 0 | EBW192156 | 1 | EBW192758 | 1 | EBW192916 | 1 | EBW193173 | 1 |
| EBW193027 | 0 | EBW192170 | 1 | EBW192763 | 1 | EBW192920 | 1 | EBW193404 | 1 |
| EBW193045 | 0 | EBW192200 | 1 | EBW192778 | 1 | EBW192921 | 1 | EBW202036 | 1 |
| EBW150046 | 1 | EBW192211 | 1 | EBW192780 | 1 | EBW192922 | 1 | EBW202045 | 1 |
| EBW150047 | 1 | EBW192331 | 1 | EBW192783 | 1 | EBW192924 | 1 | EBW202047 | 1 |
| EBW150048 | 1 | EBW192344 | 1 | EBW192789 | 1 | EBW192929 | 1 | EBW202049 | 1 |
| EBW150049 | 1 | EBW192345 | 1 | EBW192791 | 1 | EBW192930 | 1 | EBW202062 | 1 |
| EBW150058 | 1 | EBW192346 | 1 | EBW192792 | 1 | EBW192931 | 1 | EBW202067 | 1 |
| EBW150072 | 1 | EBW192347 | 1 | EBW192803 | 1 | EBW192954 | 1 | EBW202071 | 1 |
| EBW150073 | 1 | EBW192350 | 1 | EBW192807 | 1 | EBW192973 | 1 | EBW202073 | 1 |
| EBW150074 | 1 | EBW192406 | 1 | EBW192810 | 1 | EBW192977 | 1 | EBW202074 | 1 |
| EBW150075 | 1 | EBW192409 | 1 | EBW192815 | 1 | EBW192986 | 1 | EBW202075 | 1 |
| EBW150076 | 1 | EBW192416 | 1 | EBW192816 | 1 | EBW192990 | 1 | EBW202080 | 1 |
| EBW150077 | 1 | EBW192417 | 1 | EBW192817 | 1 | EBW193006 | 1 | EBW202082 | 1 |
| EBW150078 | 1 | EBW192423 | 1 | EBW192820 | 1 | EBW193026 | 1 | EBW202083 | 1 |
| EBW150084 | 1 | EBW192424 | 1 | EBW192821 | 1 | EBW193034 | 1 | EBW202084 | 1 |
| EBW150099 | 1 | EBW192430 | 1 | EBW192823 | 1 | EBW193036 | 1 | EBW202085 | 1 |
| EBW150103 | 1 | EBW192434 | 1 | EBW192827 | 1 | EBW193074 | 1 | EBW202092 | 1 |
| EBW150104 | 1 | EBW192443 | 1 | EBW192828 | 1 | EBW193099 | 1 | EBW202093 | 1 |
| EBW150110 | 1 | EBW192470 | 1 | EBW192864 | 1 | EBW193101 | 1 | EBW202102 | 1 |
| EBW150142 | 1 | EBW192488 | 1 | EBW192873 | 1 | EBW193103 | 1 | EBW202104 | 1 |
| EBW150148 | 1 | EBW192493 | 1 | EBW192878 | 1 | EBW193113 | 1 | EBW202108 | 1 |
| EBW150152 | 1 | EBW192507 | 1 | EBW192901 | 1 | EBW193123 | 1 | EBW202109 | 1 |
| EBW192005 | 1 | EBW192546 | 1 | EBW192904 | 1 | EBW193124 | 1 | EBW202113 | 1 |
| EBW192065 | 1 | EBW192556 | 1 | EBW192907 | 1 | EBW193164 | 1 | EBW202114 | 1 |
| EBW192118 | 1 | EBW192683 | 1 | EBW192909 | 1 | EBW193166 | 1 | EBW202116 | 1 |
| EBW192123 | 1 | EBW192696 | 1 | EBW192910 | 1 | EBW193167 | 1 | EBW202118 | 1 |
| EBW192151 | 1 | EBW192748 | 1 | EBW192913 | 1 | EBW193168 | 1 | EBW202170 | 1 |
| EBW202433 | 1 | EBW202436 | 1 | EBW202535 | 1 |
Table 2. Reaction of promising NVT bread wheat lines selected based on lower FHB good agronomic performance.
Conclusion
The current research finding revealed that considerable amount of the test genotypes of both PVT and NVT sets showed low infection to FHB. But it is suggested to re-evaluate further against major wheat diseases like rusts along with agronomic performance including grain yield. FHB is difficult to control, so it is imperative to prevent the disease from becoming established in a field. However, under field conditions, getting wheat lines with absolute resistance to the disease may not be a reality; thus, wheat genotypes identified in this study having minimized FHB are critical for tangible integrated fusarium controlling and contribute to sustainable disease regulator and poison inhibition.
References
Peter, R., Shewry, J., Sandra, J.H. (2015). The contribution of wheat to human diet and health. Food And Energy Security, 4(3):178-202.
FAO. (2018). Food and agriculture data. Explore Data. Crops: Wheat. Food And Agricultural Organization of The United Nations (FAO).
Kohli, M.M. (1989). Análisis De La Fusariosis Del Trigo En El Cono Sur. In: Kohli, M.M. (Ed). Taller Sobre La Fusariosis De La Espiga En América Del Sur. México, D.F. CIMMYT. CIMMYT, Asunción, Paraguay, pp:1-6.
Schroeder, H.W., Christensen, J.J. (1963). Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology, 53:831-838.
Parry, D.W., Jenkinson, P., Mcleod, L. (1995). Fusarium ear blight (scab) in small-grain cereals : A review. Plant Pathol 44:207-238.
Fokunang, C., Tembe, E., Tomkins, P. (2006). Global impact of mycotoxins on human and animal health management.
Steiner, B., Buerstmayr, M., Michael, S. (2017). Breeding strategies and advances in lin selection for fusarium headblight resistance in wheat. Tropical Plant Pathology, 42:165-174.
Schmale III, D.G., Bergstrom, G.C. (2003). Fusarium head blight in wheat. The Plant Health Instructor. DOI:10.1094/PHI-I-2003-0612-01
Engle, J., Madden, L., Lipps, P. (2003). Evaluation of inoculation methods to determine resistance reactions of wheat to fusarium graminearum. Plant Disease, 87(12):p.1530-1535.
Pereyra, S., Lori, G. (2013). Crop residues and their management in the epidemiology of fusarium head blight. In: Fusarium Head Blight In Latin America. Dordrecht: Springer, pp:143-156.
Martinez-Espinoza, A., Ethredge, R., Youmans, V., John, B., Buck, J. (2014). Identification and control of Fusarium head blight (Scab) of wheat in Georgia. University of Georgia Extension, pp:1-8.
Author Info
G.M. Abebele*Citation: Abebele, G.M. (2022). Identification of novel sources of resistance for fusarium head blight among ethiopian bread wheat germplasm. Ukrainian Journal of Ecology. 12:26-30.
Received: 01-May-2022, Manuscript No. UJE-22-62378; , Pre QC No. P-62378; Editor assigned: 03-May-2022, Pre QC No. P-62378; Reviewed: 16-May-2022, QC No. Q-62378; Revised: 20-May-2022, Manuscript No. R-62378; Published: 28-May-2022, DOI: 10.15421/2022_371
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.