Research - (2021) Volume 0, Issue 0
First record of existence of Rhinolophus ferrumequinumin in southwest of Algeria: Morphometric characterization and their ectoparasites
L. Hamida1*, R. Chaibi1, F. Benaceur1,2, A. Benchettouh3, A. Rezzoug1 and H. Gouzi1Abstract
This research is mainly concerned with the morphometric study of bats in the region of northern Sahara of Algeria and their ectoparasites. In a descriptive approach, capture outings were carried out over a period of 15 months (February 2019 and May 2020) in a natural cave called Kaf El-Maleh in the region of El-Bayadh,allowing us to count 69 individuals (34 males and 35 females) belonging to the species Rhinolophus ferrumequinum. The morphometric analysis of the population shows that the age varies between 6 and 9 years, the weight varies between 13 and 21 g and the total length varies from 8 to 10,80 cm. The results of the comparison of growth in both sexes show that females have a large size compared to males. In addition, 193 individuals of external parasites of bats were found belonging to five species (Spinturnix sp, Ornithonyssus sp, Ischnopsyllus sp, Basilia sp and Ornithodoros sp), the mites were the most abundant group (164 individuals) followed by the Ticks (12 individuals).
Keywords
Kaf El-Maleh, Chiroptera, Morphometric, Sahara.
Introduction
Chiroptera is the most manifold order of mammals having the capacity to fly (ET, 2010). It assures several roles to ecosystem as agents of pollination and seed dispersal, the prevention of the outbreaks of pests and as a good bioindicator of the quality of environment and climatic change (Jones et al., 2009). Chiroptera are threatened animals that require therefore the protection and the take of special measurements of conservation (Stebbings & Griffith, 1986). Chiroptera are potential reservoirs of emerging viral diseases in most cases zoonotic. They fly most often in urban areas (attics, cellars), by introducing pathogenic agents indoors (Calisher et al., 2006). The epidemiological importance of bats as reservoirs and transmission agents of different diseases remains the highest concern and the main reason of which; extensive studies and research has been carried out. Besides,several studies showed that bats can transmit pathogens to humans, which cause morbidity and mortality (Calisher et al., 2006). Recently, the emergence of the new coronavirus, coming probably from bats, had been reported at the beginning of March, 2019 (Fan et al., 2019). A recent study, shows that the coronavirus MERS was detected in Pipistrellus and Perimyotis (Annan et al., 2013). The same study shows that bats are the main host and the most appropriate environment for the development of viruses (Annan et al., 2013). Besides, bats are vectors of very pathogenic microbes such as Bartonella sp and Rickettsia sp (Dietrich et al., 2016).
They provide habitat for numerous ectoparasites belonging to several orders of Acari, Dermaptera, Diptera, Hemiptera and Siphonaptera (Almeida et al., 2016). Their parasites are specific to the host, due to its ecological isolation and to strategies related to the biological cycle of the parasites (Dick et al., 2003).The phenomenon of parasitism among bats remains unknown until the present day and their parasites are qualified by parasitologists as specific species.
The host-parasite relationships must be studied to inquire about the ecology of the host; this also contributes to more detailed programs of protection and conservation of biodiversity. Algeria, with its biggest area in Africa, is known by its great variety of biotopes that allow for the existence of more diversified bats. In fact, there are littoral, arid, mountainous zones, the high plains and plateaus and ancient volcanic regions. These zones can host a fauna of particular chiroptera. Bats fauna in North Africa and particularly that of Algeria is still not very well known (Ahmim, 2017).Some studies have been undertaken by many authors on chiroptera in parts of North Africa such as in Morocco, in Tunisia and Libya (Baker et al., 1974). To the best of our knowledge, several studies has been conducted on chiroptera in Algerian Northern (Ahmim, 2017; Bendjeddou et al., 2017; Loumassine et al.,; 2018; Mokrani et al., 2018).
Nevertheless, in the south of Algeria where the dominance of the arid and Saharan bioclimatic stages, ecology data on bats are very scarce and their distribution is incomplete (Korine et al., 2016). Given the importance of the surface of the Algerian desert and time when data and information on bats of arid regions and Saharan are almost nil. There is a population of Chiroptera that inhabit a cave in the region of EL-BAYD Hcalled Kaf-El Maleh.
We have attempted in this paper to contribute in the realization for the first time of a morphmetric and inventory description of the ectoparasites related to bats Rhinolophus ferrumequinum of the kaf EL Maleh located in the southwest of Algeria.
Materials and Methods
Study area
About 69 Chiroptera were collected in the cave of Kaf El-Maleh southwest of Algeria. The cave is crossed by valleys of fresh water and hyper-saline water that circulates through the cave and will be mixed with fresh water of the Oued, the presence of such a cave is in the middle of the mountain makes it more sensational and impressive.
The cave of Kef el Maleh is located in the southwest of Algeria, in 33°20′0″ and 33°23′45″N; 1°51′0″ and 1°52′15″E with an altitude of 1300 m. It takes the form of a tunnel of natural origin and hyper-saline water (Fig. 1). The cave entrance is formed by a broad natural spherical room less lived by Chiroptera that emerge to fresh air through a narrow opening, making more than 100 m of length, 2,5 m of breadth, its height is variable of 2 m in 10 m.
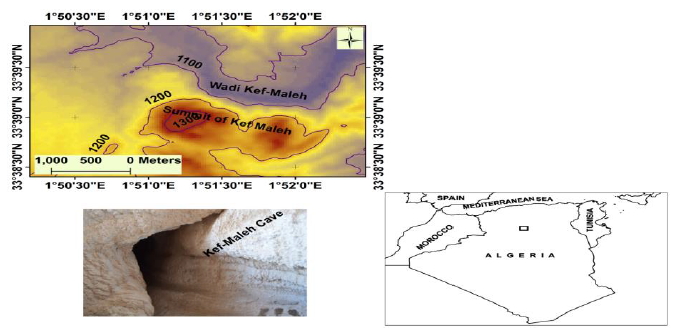
Fig 1: Map of the kef Maleh area showing the localities mentioned in the text.
Method of the capture of specimens
There are several dependable techniques of capture of different kinds of bats,in site for instance: (the capture in the Japanese net). In our case, we have used a simple and classical technique: the hand net that resembles the net of insects, with a 20 centimeter diameter and with the length of net towards 35-40 centimeter. This net is useful when bats are in a nonoperative state that is to say during the day. Due to direct contact with the Chiroptera colonies, it is necessary to carry gloves and mask to protect the face in case of sudden flight of the bats.This method requires precision and caution to obtain exact results for study.
Morphometry
To determine the present species in the cave, we haveperformed morphometric measurements, accordingto the same approach (Dietz, 2005).We can know the weight of a bat thanks to digitalscales of 500 Gram (g). For this reason, we have takena small black bag to put the bats and weigh them one after another. We have left the bats to calm down during 4 minutes then sprang balances. Finally, we have reduced the weight of the black bag to acquire the weight of the bat. With a sliding caliper, we have taken these mainmorphometric measures: the length of the body (LT), the 5th finger length (D5), the forearm length (AB), the first finger ‘thumb length (D1), tail length (LQ), total width (larg t), ear length (LO), ear width (LaO), Tibia Length (Tib), foot length (LP), the upper row teeth length (CM3), the 2nd phalanx of the 4th finger’s length (P4.2), the 1st phalanx of the 4th finger’s length (P4.1), the 3rd phalanx of the 3rd finger’s length (P3.3), the 2nd phalanx of the 3rd finger’s length (P3.2), and the 1st phalanx of the 3rd finger’s length (P3.1). In this study, the researchers shed light on the parameter of sexual dimorphism, which is observed by male and female genital apparatus.
Bats identification
Bat specie was identified by noticing morphological characters and by taking necessary measurements previously mentioned according to the key of identification (Dietz, 2005). An expert helped to identify specimens and confirmed the researcher’s identifications (see acknowledgements for more details).
Ectoparasites research
At the beginning, we have used ether soaked cotton to calm the bats, then, capturing them using different tools (brush, adhesive tape, and clamp). We have used a surgical clamp to recover the parasites on the body of the bat, an adhesive tape to recover the parasites of small size, and a toothbrush to rub the fur. Once ectoparasites were collected, we have put them in tubes of ethyl alcohol.
Ectoparasites identification
The identification is accomplished from the observation of the cited keys according to (Hopkins & Eothschild,1953; Stanyukovich, 1997; Hiregaudar et al., 1956; Guimaraes & D'Andretta, 1956; Guerrero, 1993; Battesti et al., 2006; Peterson and Hurka, 1974).
The analysis
The statistical analyses were performed using Excel computer programs for the entrance of data, and Excel stat and STATISTICA for the data processing.
Principal component analysis (PCA)
Principal component analysis (PCA) is mainly used as a tool of exploratory analysis of data, that is generally dimensional. We have performed the Principal component analysis (PCA) for a better knowledge of relations between the parameters of growth.
Results and Discussion
The results of this research revealed the presence of single species of bat Rhinolophus ferrumequinum. In order to obtain significant results linked to the various measurements carried out on the 69 individuals collected, on which we have carried out morphometric measurements, as mentioned above. The surveyed population consists of 34 males and 35 females with an average age of 7,48 years ranging from 6 to 9 years (Table 1). The average weight is 14.92 ± 1.95. It varies from 13 to 21 g. The overall length varies from 80 to 108,0 mm. The average length is 89.4 ± 0.64 mm, the length of AB ranges from 50 to 59 mm on average is 54 ± 0.23. Measurements made at the level of the ear give very variable values ranging from 19 to 30 mm for the length of the ear, with an average of 20.8 ± 0.16 mm.The Length of the upper row of teeth (cm3 )constitutes the last analyzed morphometric criterion, their measurements give very variable values ranging from 5 mm to 10 mm, with an average of 8.7 ± 0.16 mm.
| Variable* | N | Mean | Mode | Frequency of Mode | Minimum | Maximum | Lower Quartile |
Upper Quartile |
Std.Dev |
|---|---|---|---|---|---|---|---|---|---|
| Weight (gram) | 69 | 14.92 | 14.00 | 20 | 13.00 | 21.00 | 13.00 | 16.84 | 1.95 |
| Age (year) | 69 | 7.48 | 8.000 | 32 | 6.00 | 9.00 | 7.00 | 8.00 | 0.82 |
| Length (cm) | 69 | 8.94 | 8.800 | 17 | 8.00 | 10.80 | 8.50 | 9.00 | 0.64 |
| Width (cm) | 69 | 36.16 | 37.00 | 12 | 31.41 | 38.00 | 36.00 | 37.00 | 1.38 |
| AB* (cm) | 69 | 5.40 | 5.400 | 33 | 5.00 | 5.90 | 5.30 | 5.40 | 0.23 |
| D5** (cm) | 69 | 6.95 | 6.700 | 21 | 5.50 | 8.00 | 6.70 | 7.10 | 0.44 |
| D3+ (cm) | 69 | 8.30 | 8.800 | 15 | 7.00 | 8.80 | 8.10 | 8.70 | 0.40 |
| D1++ (cm) | 69 | 0.55 | .5000 | 23 | 0.40 | 1.00 | 0.50 | 0.60 | 0.14 |
| Tib (cm) | 69 | 2.38 | 2.400 | 22 | 1.70 | 2.90 | 2.30 | 2.50 | 0.19 |
| LP (cm) | 69 | 0.95 | .9000 | 37 | 0.90 | 1.00 | 0.90 | 1.00 | 0.05 |
| LO (cm) | 69 | 2.08 | 2.000 | 22 | 1.90 | 3.00 | 2.00 | 2.10 | 0.16 |
| LaO (cm) | 69 | 1.40 | 1.400 | 27 | 1.20 | 1.80 | 1.40 | 1.50 | 0.12 |
| P3.2 (cm) | 69 | 2.95 | 3.000 | 34 | 1.30 | 3.20 | 2.80 | 3.00 | 0.23 |
| P3.3 (cm) | 69 | 0.31 | .3000 | 64 | 0.30 | 0.80 | 0.30 | 0.30 | 0.06 |
| P4.1 (cm) | 69 | 1.00 | 1.000 | 43 | 0.90 | 1.20 | 1.00 | 1.00 | 0.08 |
| P4.2 (cm) | 69 | 1.86 | 1.800 | 34 | 1.10 | 2.00 | 1.80 | 1.90 | 0.12 |
| CM3 (cm) | 69 | 0.87 | .9000 | 45 | 0.50 | 1.00 | 0.90 | 0.90 | 0.10 |
| P3.1 (cm) | 69 | 1.71 | 1.600 | 26 | 1.40 | 1.90 | 1.60 | 1.80 | 0.12 |
| LQ (cm) | 69 | 3.22 | 3.000 | 29 | 3.00 | 3.90 | 3.00 | 3.40 | 0.22 |
Table 1. Descriptive statistics of the sample.
Comparison between both sexes
The results obtained are summarized in Table 2 and Fig. 2. We find that females have a large size compared to males. In fact, there is a highly significant difference between females and males from the point of view of size.
| Variable | Mean females | Mean males | t-value | df | p | Valid N. females |
Valid N. males |
Std.Deviation females | Std. Deviation males |
|---|---|---|---|---|---|---|---|---|---|
| Weight (gram) |
15.66 | 14.17 | 3.41 | 67 | 0.001 | 35 | 34 | 1.85 | 1.77 |
| Length (centimetre) | 8.93 | 8.94 | -0.10 | 67 | 0.92 | 35 | 34 | 0.57 | 0.72 |
| Width (centimetre) | 35.89 | 36.45 | -1.72 | 67 | 0.90 | 35 | 34 | 1.60 | 1.06 |
Table 2. Comparison between men and women in terms of length, weight, width (T-tests; Grouping:Sex Group 1:females Group 2:males).

Fig 2: Comparison between both sexes. 1-Box plot of weight grouped by sex; 2-Box plot of length grouped by sex; 3-Box plot of width grouped by sex.
We noticed that there is no statistically significant difference between the length averages of males and females (Table 2, Fig. 2).
The box-plot shows that there is more uniformity in width in males than in females. However, there is no significant difference between the averages of the two groups (Fig. 2, Table 2).
ACP analysis
The results of principal component analysis for males are showed in Fig. 3. The axle F1 is apparently linked in variables (AB), (LP), width and (P3.1), while the axle F2 is principally linked to P(4.1). On the factorial design F2, 27, 49% of information can be interpreted according to this design, Age is positively correlated with the weight, total width and P(3.2). On the other hand, a weak correlation was observed between this variable and P(4.2). However, according to the Fig. 3A, it is readable that the (largt) is positively correlated with the variables; (AB), D3, P(4.2) and negatively with cm3. Thus, we observed there is a negative correlation between LO and the variables (AB), (LP), P(3.2), P(4.2) and P(3.1). On the other hand, it is positively correlated with the cm3 and the LQ, and weakly correlated with the other parameters.
Besides, the results ACP results for females are given in (Fig. 3B). It clearly appeared that the sex is correlated with the two axes F1 and F2. In fact, we could deduce that the variable P(3.3) is positively correlated with the Weight, D1, LO and the LQ, while it is negatively correlated with the AB, D3, and Tib, which can be seen on the correlation circle on the F1 and F2 axes. Thus, on the factorial design F1 (34,08%), we observed that the LO is positively correlated with the P(3.3), LQ and LaO. Hence, a positive correlation between the AB with the D5, D3, Tib and the P(4.2) was observed. Nevertheless, a negative correlation with the LO, LaO and the P(3.3) was found.
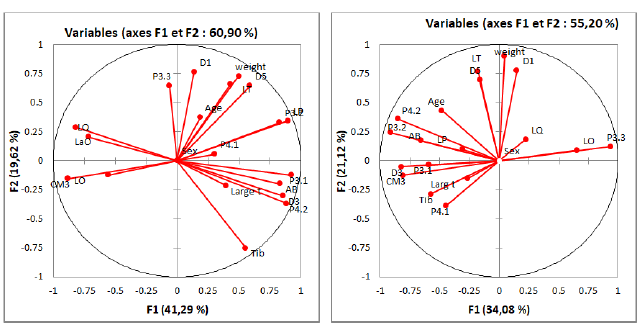
Fig 3: The ACP parameters studied for all males of Rhinolophus ferrumquinum.
The ACP parameters studied for all females of Rhinolophus ferrumquinum
Inventory of the bat ectoparasites
A total of 69 specimens of the Rhinolophus ferrumequinum bat were subjected to a parasitological investigation which enabled us to collect (n=193) ectoparasite individuals belonging to five species (Spinturnix sp, Ornithonyssus sp, Ischnopsyllus sp, Basilia sp and Ornodoros sp), in which one Bat fly species (Nycteribiidae), a single bat flea (Ischnopsyllidae), two moth species (Spinturnix sp, Ornithonyssus sp) and one tick species (Argasidae). Moths were the most abundant group with 164 individuals collected. Ticks were the second most abundant with (12) individuals and fleas were the rarest group with 7 individuals (Table 3).
| Parasite | Number | Family |
|---|---|---|
| Spinturnix sp | 160 | Spinturnicidae |
| Ornithodoros sp | 12 | Argasidae |
| Ischnopsyllus sp | 10 | Ischnopsyllidae |
| Basilia sp | 7 | Nycteribiidae |
| Ornithonyssus sp | 4 | macronyssidae |
Table 3. Ectoparasites and their number on Rhinolophus ferrumequinum in the studied cave.
Chiroptera is among the least explored populations despite their importance in the functioning of ecosystems, especially around the south of Algeria. They are nocturnal animals and found in caves that are difficult to reach which explain the lack of information morphometric and parasitic fauna associated with Rhinolophus ferrumequinum. The results of the morphometric study of Rhinolophus ferrumequinum showed that females have a larger size compared to males. Our results are in agreement with the study of Albayrak et al. (2013) on many other bat species of Rhinolophus ferrumequinum from Turkey which show that the mean values of total length and total weight are greater in females than in males.
According to results reported by Jiang et al. (2019) who reported a study on the possible causes of the variation in the body size of the Greater Horseshoe Bat in Jilin Province, China, where they have reported that the body weight of the greater Horseshoe Bat female is superior to that of males in some sites but in some sites they have found the opposite.
The average value of the total length measured in our study (89.4 ± 0.64mm) is greater than previously published by (Zagorodniuk, 1999)for specimens of Rhinolophus ferrumequinum from Eastern Europe with average value of the total length 62,5 (± 4.3) mm in the Carpathian region and 60,8 (± 4.7 mm) in the Crimearegion. For weight results,our results (14.92 ± 1.95 g) are a little lower than those noted by Jiang et al.(2019) who recorded a body weight between 15.6 to 29.3 g in female Rhinolophus ferrumequinum and 16.2 to 25.6 g for males.
In addition, the length results (AB) varies between 50 to 59 mm with average value equals to 54 ± 0.23 mm while P(4.1) varies between 9 to 12 mm,average=10, P4.2 varies between 11 to 20 mm Average=18.6 mm.
Our values are in agreement with those found by Dietz et al.(2006) who studied the variation in measurement of five species of rhinolophidae in Europe which shows that in, Rhinolophus ferrumequinum, the length of the forearm (AB ) varies between 53.0 to 60.5 mm Average = 57.0 mm for males and from 58.3 to 53.7 mm for females Never the less, the length of P(4.1) varies from 9.5 to 12.7 mm for male’s average of 11.1 mm and 9.5 ton 13.4 mm for females while P(4.2) length varies from 18.3 to 21.7 mm, average 19.8 mm in males and 16.0 to 22.5 mm average 20.1 for females.
Or the length of the tail “LQ” varies between (30 to 39 mm) average 32.2 mm, Wingspan varies from 314 to 380 mm average 361.6 mm, Measurements made at the level of the upper molars “CM3” 5 mm to 10 mm, with an average of 8.7 ± 0.16 mm and the measurements taken at the level of the ear give very variable values ranging from 19 mm to 30 mm for the Length of the ear “Lo”, with an average of 20.8 ± 0.16 mm. The results of measurements of total length, ear length and tail length of horseshoe bats were similar with study of Albayrak et al. (2013).
Zagorodniuk (1999) has made a study on horseshoe bats in Eastern Europe, he recorded the following body length measurements (52.0 to 71.0 mm) for Carpathian species, (50.0 to 73.0 mm) for species of Crimea are less than what we found, in terms of tail length and ear length. These are the same lengths we have found. Dietz (2007) has reported that the lengths in the largest of the five horseshoe bats, AB>54 mm (54.0-62.4 mm, lowest extreme 51.0 mm, D5: 63-77 mm, D3: 78-94 mm, P4.1: 9.5-13.4 mm; P4.2: 17.5-22.5 mm).
The morphometric measurements of Rhinolophus Ferrumequinum for both sexes taken Al-Ramadan et al. (2020) in the central and coastal regions ofSyria vary from 90 to 91 mm for total length, taillength varies from 36 to 37 mm, ear length is 1.1 mm,wingspan varies from 349 to 350 mm and the forearmlength varies from 55 to 56 mm.
The age investigation of the Rhinolophus ferrumequinum in our study was varied between 6and 9 years. The Greater Horseshoe Bat who had been controlled by Caubere et al., (1984) where they are found his age of at least 30 years and 6 months.
The captured species is 16 years and five months old.,According to Budinski et al.(2015), the growth and development of bats and their morphology are influenced by climatic factors Krystufek (1993) shows that the R. ferrumequinum which inhabit the hot zones are large in size whereas the species which come in the cold zones are smaller. Hence,infections by ectoparasites can have significant consequences for the bats that carry them (Holz et al., 2018).For this, the ectoparasites associated with bats have been studied. Parasitological examination of the different pathogenic individuals allowed us to collect (n=193) ectoparasite individuals belonging to five species (Spinturnix sp, Ornithonyssus sp, Ischnopsyllus sp, Basilia sp and Ornithodoros sp). Some Rhinolophus ferrumequinum are much more infested than others, the most parasitized being, as is to be expected, the species whose gregarious behavior is more marked. Flies were the rarest group with 7 individuals, (Marshall, 1976) noted that in the parasitic fly the specificity decreases due to the ease of obtaining new host species. In a previous study on ectoparasites of bats in Algeria 12 (Bendjeddou et al., 2017) have shown the presence of ectoparasites belonging to different groups (Nycteribiidae, Streblidae, Siphonaptera, Heteroptera, Mesostigmata, Argasidae, and Ixodidae) but it did not find some species that we have found in our study (Ornithonyssus sp, Ornithodoros sp). Our results are consistent with previous work stating that Rhinolophus ferrumequinum is the host of the genus Spinturnix (Imaz et al., 1999; Krištofik & Danko, 2012) but our data do not agree with the study made by (Orlova and Orlov, 2018) which indicated the presence of other species of ectoparasites (Rhinolophopsylla unipectinata,Phthiridium biarticulatum) in the genus Rhinolophus, we believe that this difference could be justified by the fact that our study would have worked on the same genus but a different species. The genus Basilia has been found in bats (Hassan et al., 2010).
Also, mites and Diptera are known for their ability to parasitize bats (Bezerra and Bocchiglieri, 2018). According to (Holz et al., 2018), the bald–smiled (Rhinolophus rouxi) of Sri Lanka accommodate the following ectoparasites: Nycteribiidae (Diptera), Streblidae (Diptera), Argasidae (Acarina, Ixodidae (Acarina), Spinturnicidae (Acarina), Trombiculidae (Acarina), Leeuwenhoekiidae (Acarina).
Thus, even authors show that the abundance of the flies, of dust mites and the ticks different between kinds from bald-smiled and their case affected by season. Also environmental conditions, can significantly affect parasite abundance. The specimens of ectoparasites were compared with those given by Khelfaoui et al.(2018) who carried out a study on the parasites in different localities of Numidia, eastern Algeria, Where they are found Ixodes vespertilionis, Brachytarsina flavipennis, Phthiridium biarticulatum, Nycteribia pedicularia in Rhinolophus ferrumequinum.
Thus, even authors show that the abundance of the flies, of dust mites and the ticks different between kinds from bald-smiled and their case affected by season. Also environmental conditions, can significantly affect parasite abundance. The specimens of ectoparasites were compared with those given by Khelfaoui et al.(2018) who carried out a study on the parasites in different localities of Numidia, eastern Algeria, Where they are found Ixodes vespertilionis, Brachytarsina flavipennis, Phthiridium biarticulatum, Nycteribia pedicularia in Rhinolophus ferrumequinum.
Conclusion
In the last decade, little attention has been given to animals such as bats in the Mediterranean basin and especially in Algeria. This work fills the gaps of informations on this faunal group whose ecological importance is proven. Indeed, through this work, we reported for the first time a complete study on morphometric characteristics and parasitofauna of genus Rhinolophus in the southwest of Algeria (EL Bayadh). A total of 69 bats of the genus Rhinolophus were captured, including 34 males and 35 females. Thus, A positive correlation was noticed between Weight and total length for both male and female. Besides, age range of the Horseshoe Bat was between 6 years and 9 years. In addition, the investigation of bats ectoparasites was carried out and five genera of parasites (Spinturnix sp, Ornithonyssus sp,Ischnopsyllus sp, Basilia sp and Ornodoros sp) including 4 taxonomic groups was found. Hence, further studies on genus Rhinolophus are recommended to understand its functional attributes, its interaction and ecosystem in order to protect and preserve these faunal groups by creating protected areas.
Acknowledgements
We thank our colleagues ≪Hiba Loumassine, Agence Auvergne-Rhone-Alpes, Lyon, France≫ and ≪ Boualem Fadia, ecole nationale superieur, Lagouat, Algeria≫ for their assistance and comments that greatly improved the manuscript. This work was supported by the Algerian General Directorate of Scientific Research and Technological Development (DGRSDT).
References
Ahmim, M. (2017). Current status, distribution and conservation status of Algerian bats (Mammalia: Chiroptera). Journal of Threatened Taxa, 9:9723-9733.
Albayrak, İ., Pamukoğlu, N., Baydemir, N.A. (2013). Taxonomic Status and Karyotype of Rhinolophus ferrumequinum Schreber, 1774 from Turkey Rhinolophidae, Chiroptera. Hacettepe Journal of Biology and Chemistry, 41:235-241.
Almeida, J.C.D., Martins, M.A., Guedes, P.G., Peracchi, A.L., Serra-Freire, N.M. (2016). New records of mites (Acari: Spinturnicidae) associated with bats (Mammalia, Chiroptera) in two Brazilian biomes: Pantanal and Caatinga. Revista Brasileira de Parasitologia Veterinária, 25:18-23.
Al-Ramadan, Y., Ibrahim, N., Al-Omar, A. (2020). Documenting some species of bats in the central and coastal regions of Syria. International Journal Science Research in Biological Sciences.
Annan, A., Baldwin, H.J., Corman, V.M., Klose, S.M., Owusu, M., Nkrumah, E.E., Kalko, E.K. (2013). V, Lina, PHC, Godlevska, E.V, Reusken, C., Seebens, A., Gloza-rausch, F., Vallo, P., Tschapka, M., Drosten, C., Drexler, JF, pp:456-459.
Baker, R.J., Davis, B.L., Jordan, R.G., Binous, A. (1974). Karyotypic and morphometric studies of Tunisian mammals: bats.
Battesti, D.M., Arzua, M., Bechara, G.H. (2006). Carrapatos de importancia medicoveterinaria (Mammalia: Chiroptera) del Nuevo Mundo I. Clavepara los generos y Nycterophiliinae. Acta Biologica Venezuelica, 14:61-75.
Bendjeddou, M.L., Loumassine, H.A., Scheffler, I., Bouslama, Z., Amr, Z. (2017). Bat ectoparasites (Nycteribiidae, Streblidae, Siphonaptera, Heteroptera, Mesostigmata, Argasidae, and Ixodidae) from Algeria. Journal of Vector Ecology, 42:13-23.
Bezerra, R.H.S., Bocchiglieri, A. (2018). Association of ectoparasites (Diptera and Acari) on bats (Mammalia) in a restinga habitat in northeastern Brazil. Parasitology Research, 117:3413-3420.
Budinski, I., Jojić, V., Jovanović, V.M., Bjelić-Čabrilo, O., Paunović, M., Vujošević, M. (2015). Cranial variation of the greater horseshoe bat Rhinolophus ferrumequinum (Chiroptera: Rhinolophidae) from the central Balkans. Zoologischer Anzeiger-A Journal of Comparative Zoology, 254:8-14.
Calisher, C.H., Childs, J.E., Field, H.E., Holmes, K.V., Schountz, T. (2006). Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews, 19:531-545.
Caubère, B., Gaucher, P., Julien, J.F. (1984). Un record mondial de longévité in natura pour un chiroptère insectivore?. Revue d'écologie.
Christe, P., Arlettaz, R., Vogel, P. (2000). Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecology Letters, 3:207-212.
Dick, C.W., Gannon, M.R., Little, W.E., Patrick, M.J. (2003). Ectoparasite associations of bats from central Pennsylvania. Journal of Medical Entomology, 40:813-819.
Dietrich, M., Tjale, M.A., Weyer, J., Kearney, T., Seamark, E.C., Nel, L.H., Markotter, W. (2016). Diversity of Bartonella and Rickettsia spp. in bats and their blood-feeding ectoparasites from South Africa and Swaziland. PLoS One, 11:e0152077.
Dietz, C. (2005). Illustrated identification key to the bats of Egypt. Electronic Publication.
Dietz, C., Dietz, I., Siemers, B.M. (2006). Wing measurement variations in the five European horseshoe bat species (Chiroptera: Rhinolophidae). Journal of Mammalogy, 87:1241-1251.
Dietz, C. (2007). Aspects of ecomorphology in the five European horseshoe bats (Chiroptera: on bats (Mammalia) in a restinga habitat in northeastern Brazil. Parasitol Res 117:3413-3420.
ET, Synth. (2010). Les chauves-souris arboricoles en situation précaire au Québec. Le Naturaliste Canadien.
Fan, Y., Zhao, K., Shi, Z.L., Zhou, P. (2019). Bat coronaviruses in China. Viruses, 11:210.
Guerrero, R. (1993). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo I. Clave para los géneros y Nycterophiliinae. Acta Biologica Venezuelica, 14:61-75.
Hassan, V., Zakkyeh, T., Mozafar, S., Alireza, M., Maryam, K., Mojtaba, T. (2010). Ectoparasites of lesser mouse eared bat, Myotis blythii from Kermanshah Iran. Asian Pacific Journal of Tropical Medicine, 3:371-373.
Hiregaudar, L.S., Bal, D.V. (1956). Some ectoparasites of bats from India. Agra University Journal of Research (Science), 5:1-134.
Holz, P.H., Lumsden, L.F., Hufschmid, J. (2018). Ectoparasites are unlikely to be a primary cause of population declines of bent-winged bats in south-eastern Australia. International Journal for Parasitology: Parasites and Wildlife, 7:423-428.
Hopkins, G.H.E., Eothschild, M. (1953). An illustrated catalogue of the rothschild collection of fleas (siphonaptera) in the British Museum (Natural History) with keys and short descriptions for the identification of families, genera, species and subspecies. Tungidae and Pulicidae. An Illustrated Catalogue of the Rothschild Collection of Fleas (Siphonaptera) in the British Museum (Natural History) with Keys and Short Descriptions for the Identification of Families, Genera, Species and Subspecies.
Imaz, E., Aihartza, J.R., Totorika, M.J. (1999). Ectoparasites on bats (Gamasida, Ixodida, Diptera) in Biscay (N Iberian peninsula). Miscel lània Zoològica, pp:21-30.
Jiang, T., Wang, J., Wu, H., Csorba, G., Puechmaille, S.J., Benda, P., Feng, J. (2019). The patterns and possible causes of global geographical variation in the body size of the greater horseshoe bat (Rhinolophus ferrumequinum). Journal of Biogeography, 46:2363-2377.
Jones, G., Jacobs, D.S., Kunz, T.H., Willig, M.R., Racey, P.A. (2009). Carpe noctem: the importance of bats as bioindicators. Endangered Species Research, 8:93-115.
Loumassine, H.E., Allegrini, B., Bounaceur, F., Peyre, O., Aulagnier, S. (2018). A new mammal species for Algeria, Rhinopoma microphyllum (Chiroptera: Rhinopomatidae): morphological and acoustic identification. Mammalia, 82:85-88.
Marshall, A.G. (1976). Host-specificity amongst arthropods ectoparasitic upon mammals and birds in the New Hebrides. Halcyon.
Mokrani, Y., Mimeche, F., Nouidjem, Y., Saheb, M. (2018). Rapid assessment of cave-dwelling bat diversity in the Chebket ES-Sellaoua Mountains (Eastern Algeria). Arxius de Miscel lània Zoològica, 16:112-120.
Orlova, M.V., Orlov, O.L. (2018). Contribution to the ectoparasite fauna of bats (Chiroptera: Vespertilionidae, Rhinolophidae) of Crimea. Entomological Review, 98:319-323.
Peterson, B.V., Hurka, K. (1974). Ten new species of bat flies of the genus Trichobius (Diptera: Streblidae). The Canadian Entomologist, 106:1049-1066.
Stebbings, R.E., Griffith, F. (1986). Distribution and status of bats in Europe. Institute of Terrestrial Ecology.
Khelfaoui, F., Kebaci, A., Benyacoub, S. (2018). New data on insecta and acarina parasitizing bats (Mammalia: Chiroptera) in Numidia, eastern Algeria. Bulletin Social Zoology Fr, 143:63-73.
Korine, C., Pilosof, S., Gross, A., Morales-Malacara, J.B., Krasnov, B.R. (2017). The effect of water contamination and host-related factors on ectoparasite load in an insectivorous bat. Parasitology Research, 116:2517-2526.
Krištofík, J., Danko, S. (2012). Arthropod ectoparasites (Acarina, Heteroptera, Diptera, Siphonaptera) of bats in Slovakia. Vespertilio, 16:167-189.
Stanyukovich, M.K. (1997). Keys to the gamasid mites (Acari, Parasitiformes, Mesostigmata, Macronyssoidea et Laelaptoidea) parasitizing bats (Mammalia, Chiroptera) from Russia and adjacent countries. Rudolstädter Naturhistorische Schriften, 7:13-46.
Zagorodniuk, I.V. (1999). Taxonomy, biogeography and abundance of the horseshoe bats in Eastern Europe. Acta Zoologica Cracowiensia, 42:407-421.
Author Info
L. Hamida1*, R. Chaibi1, F. Benaceur1,2, A. Benchettouh3, A. Rezzoug1 and H. Gouzi12Research Unit of Medicinal Plant (RUMP,3000 Laghouat) Attached to Center of Biotechnology (CRBt, 3000, Constantine), Algeria
3Department of Agricultural Sciences, Amar Thelidji University, Laghouat (UATL), 03000, Algeria
Citation: Hamida, L., Chaibi, R., Benaceur, F., Benchettouh, A., Rezzoug, A., Gouzi, H. (2021). First record of existence of Rhinolophus ferrumequinumin in southwest of Algeria: Morphometric characterization and their ectoparasites. Ukrainian Journal of Ecology 11 (9), 159-166.
Received: 08-Nov-2021 Accepted: 30-Nov-2021 Published: 08-Dec-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
