Research - (2023) Volume 13, Issue 2
First checklist of benthic macroinvertebrate communities from Chrea National Park, Blida province, Northern Algeria
R. Matallah1*, K. Rabhi2, M. Boumaaza2 and R. El-Farroudji3Abstract
Aquatic macroinvertebrates are an important component of biodiversity in stream Ecosystems. Conserving and enhancing freshwater biodiversity is important for maintaining ecosystem integrity and sustainability. This requires understanding how the composition of biological communities is influenced by environmental factors at different scales. Factors such as water chemistry, temperature, flow, and land use can all affect the distribution and abundance of different species in freshwater ecosystems. The North of Algeria has a complex hydrographic network, but the inventory of aquatic macroinvertebrates community remains incomplete.
This study aimed at compiling an inventory of the macroinvertebrate inhabiting the aquatic ecosystems of each stations in Chréa National Park (province of Blida, Algeria). The results were used to answer two questions: (i) macroinvertebrate composition and richness in these protected ecosystems? (ii) Which taxon or group of taxa is the most dominant?.
Different sampling methods (Surber, kick samplers and artificial substrate) were used depending on the characteristics of the sites chosen.
The sampling was carried out during three months (February to April, 2021). It was analyzed and identified 1345 organisms belonging to 41 macroinvertebrate families. The system showed high richness and diversity of organisms in response to water quality. The presence of Leptophlebiidae, Baetidae, Heptageniidae and Chironomidae with high abundance of the families showed the potential as biological indicators of a clean ecosystem.
To collect the samples, It was used a surber sampler 1 mm mesh every week, with six repetitions in each station, from February to April, 2021. After cleaning and removal of debris. we proceeded by a pre-sorting in morpho-species, then a sorting of the different taxa was carried out in the laboratory. the organisms were identified using a stereoscopic microscope and identification keys. The community structure including abundance, richness and composition was statistical different between stations.
Keywords
Benthic macroinvertebrate, Abundance, Diversity, Chréa national park, Aquatic ecosystem.
Introduction
The hydrostreams are among the most complex and dynamic ecosystems. They have essential roles in the conservation of biodiversity, in the functioning of organisms and in the cycle of organic matter. Most rivers have suffered from anthropogenic effects: regression of species, decrease in fish populations, depletion of groundwater, degradation of water quality, increasingly frequent and intense floods (Palatov and Rajabov, 2017, Everard and Powell, 2002, Dynesius and Nilsson, 1994).
In northern Algeria, the complexity of the hydrosystems and the difficult climatic conditions (decline in rainfall, high temperature) have led to the increasing fragmentation of environments reflected in deep and rapid changes invertebrate communities with loss of diversitys (Lounaci, 2005).
In Algeria, populations of benthic Macroinvertebrates remain poorly known despite some studies initiated on the rivers of northwestern Algeria (Djitli, et al., 2021, Saal, et al., 2020, Baaloudj, et al., 2020, Mimeche, et al., 2019, Djamai, et al., 2019, Benzina and Bachir, 2018, Sellam, et al., 2017).
The literature reveals that there is no scientific study carried out on the benthic macroinvertebrates of the Chiffa river, so this study was conducted to establish a first list of taxa inhabiting the courses at the level of the Chréa National Park.
One of the largest national Parks of Algeria is located in Blida Province, named after Chréa, a town near this park. The Chréa National Park is located in the Blida Province of the North West Region of Algeria. The park incorporates the mountainous area of the Blidean Atlas, part of the Tell Atlas rang. The hydrographic network of Chrea National Park is an ecosystem characterized by its great capacity to capture and store water, because many of rivers have their headwaters and sources in them (Fella Hamaidi-Chergui, et al., 2013).
Benthic macroinvertebrates are easy to collect and have relative easy identification, having diversified responses to different levels of disturbance, reflecting the state of the whole aquatic ecosystem (Reice and Wohlenberg, 1993).
The Chiffa River are home to a number of plant and animal species that depend on a clean and productive aquatic environment. The insects, worms, and mollusks found in rivers are excellent bioindicators of habitat quality and physical conditions in aquatic systems (Tahar, et al., 2021). These organisms, collectively known as aquatic macroinvertebrates, play a crucial role in organic material processing and are a major food source for fish. According to the River Continuum Concept, the physical conditions along natural streams change in a predictable pattern that should translate into gradients in the aquatic biological community.
The hydrographic network has historically been subject to human interference via logging, highways, and development. These activities can negatively impact the river environment and aquatic organisms; however restoration efforts have been underway.
The aim of this tudy is to characterize the species composition, abundance, and diversity of the assemblages of the benthic macroinvertebrate communities living in some hydrosystems of this protected area.
Materials and Methods
Study area
Oued Chiffa is located between the provinces of Medea and Blida. This waterstream crosses a protected area and receives effluents at different points along its course. She takes her source in the Blidian Atlas in North of Medea, and unites with Oued el Hamdania and Oued el Merdja at the level of the town of El Hamdania at the point (36°22'36.9"N 2°46'28.5"E) then extends to flow into the Mediterranean sea. Its course crosses the Chréa National Park and extends into the mountainous area. The study was carried out on three sites which were chosen according to their location and therefore their topography (Fig. 1).
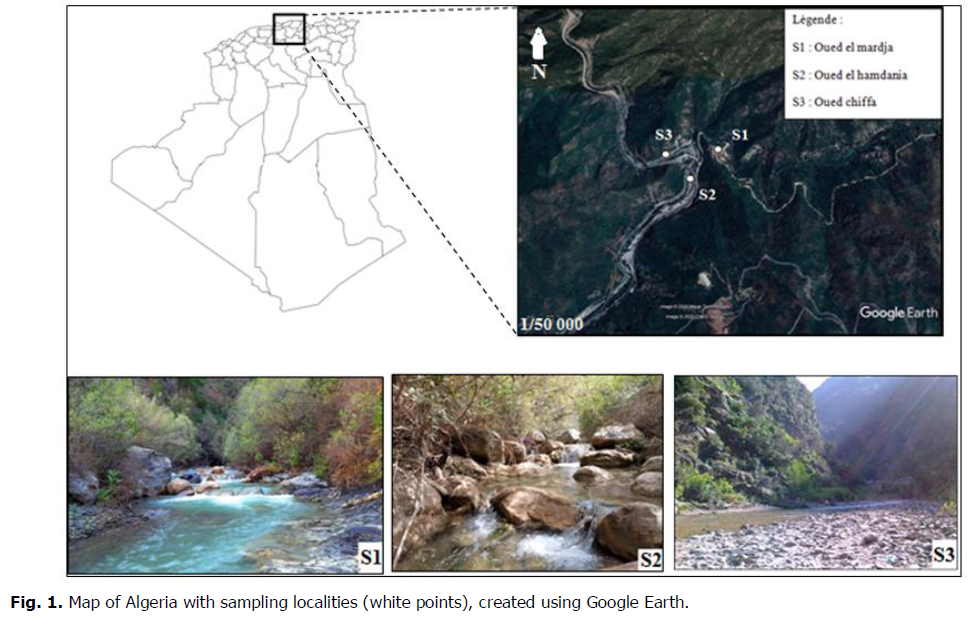
Fig 1. Map of Algeria with sampling localities (white points), created using Google Earth.
Site 1 is located upstream on the Oued el Merdja. The average width of the river bed is 9m, the geographical position is 36°22'37.2"N 2°46'28.6"E.
Site 2 is located at Oued El Hamdania, has an average width of 7 m (dominated by pebbles) and the geographical position is 36°22'36.5"N 2°46'28.9"E.
Site 3 on Oued Chiffa is a site near the road with an average bed width of 25 m and the geographical position 36°22'36.7"N 2°46'27.4"E. The sampling sites included different elevation ranges with the lowest S3 at 370 m followed by S1 with 400 m elevation and the highest S2 at 450 m.
The study area has an irregular rainfall pattern that characterizes the Mediterranean climate, with a period low rainfall which includes the months of May to October, and a more at least wet period between November and April. his pelvis located in an area with a sub-humid climate. The region is primarily forestry.
Sampling and analysis methods
Specimens were collected in freshwater habitats. Most of the biological material for this research was collected during three months sampling period from February to April 2020. samples were taken weekly, totalizing ten samples in each station.
To assess the diversity of macroinvertebrates in the entire stream, it is important to sample the stream margin and other low flow habitats (Roy et al., 2003). Sampling effort was optimized by performing all possible microhabitats.
The easiest method to obtain purely quantitative samples is with a Surber Sampler. This device only works in riffle and run habitats. Benthic collections using a Surber type sampling device must be taken from shallow riffles and/or runs. Because flowing water carries macroinvertebrates into the collecting net as the benthos is disturbed, this sampling device requires specific velocity and depth conditions to work properly.
Obviously, samplers of different sizes can accommodate different conditions (e.g., smaller samplers sometimes work better in shallow headwater streams with low velocity) (Ghani, et al., 2016).
Macroinvertebrate were collected using a surber sampler or picked manually from rock and pebbles, while imagos and adult of some groups like beetles were caught with a hand net.
The benthic material is transferred to a sieve (hole size smaller than or equal to the net on the sampling device). A wash bottle is used to rinse the invertebrates and smaller debris from the larger particles. The sample is then transported from the sieve to a collection container (Stark, 1993).
Samples must be labeled properly and sampled organisms were sent to the laboratory, where screening was done and the specimens were preserved in ethanol 70%. After that, Collected specimens were studied under a Leica M205 and Olympus stereomicroscope. The organisms were grouped into morpho-species and identified until the last taxonomic level that was possible.
The majority of specimens were collected at the larval stage, but a small number of adults were caught also. The identification has been performed and checked by using appropriate literature (Bilardo and Rocchi, 2011, Moisan, 2010, Bouchard, 2004, Tachett, et al., 2002, Heidemann and Seidenbusch, 2002).
Diversity was evaluated through species richness (S), Shannon diversity index (H’), Pielou evenness index (equitability) (J), and Simpson dominance index (D), according to Magurran (1988). Finally, In order to compare the degree of similarity between the stations, the Jaccard coefficient (J) was used.
Results
Faunistic composition
In sampling sites, a total of 1345 individuals of benthic macroinvertebrate larvae and adults were collected in different habitats. The different taxa collected are reported in the table below (Table 1).
| Phylum | Class | Order | Family | S1 | S2 | S3 | Total effectif |
|---|---|---|---|---|---|---|---|
| Arthropoda | Insecta | Trichoptera | Hydropsychidae | 50 | 1 | 9 | 60 |
| Rhyacophilidae | 1 | 0 | 4 | 5 | |||
| Leptoceridae | 1 | 0 | 0 | 1 | |||
| Hydroptilidae | 3 | 0 | 1 | 4 | |||
| Odonatoptera | Libellulidae | 12 | 1 | 0 | 13 | ||
| Coenagrionidae | 3 | 0 | 1 | 4 | |||
| Conduliidae | 0 | 0 | 4 | 4 | |||
| Synthemistidae | 0 | 1 | 0 | 1 | |||
| Ephemeroptera | Leptophlebiidae | 1 | 0 | 4 | 5 | ||
| Baetidae | 4 | 13 | 65 | 82 | |||
| Hyptageniidae | 3 | 0 | 0 | 3 | |||
| Oligoneuriidae | 3 | 0 | 0 | 3 | |||
| Caenidae | 25 | 94 | 62 | 181 | |||
| Ameletidae | 1 | 0 | 0 | 1 | |||
| Isonychiidae | 1 | 2 | 1 | 4 | |||
| Lepidoptera | Cambidae | 0 | 0 | 1 | 1 | ||
| Plecoptera | Perlidae | 12 | 0 | 2 | 14 | ||
| Leuctrdae | 0 | 0 | 1 | 1 | |||
| Chloroperlidae | 3 | 0 | 0 | 3 | |||
| Capniidae | 1 | 0 | 0 | 1 | |||
| Diptera | Tipulidae | 42 | 4 | 9 | 55 | ||
| Chironomidae | 6 | 215 | 71 | 292 | |||
| Simulidae | 0 | 75 | 31 | 106 | |||
| Adult (NI) | 0 | 0 | 1 | 1 | |||
| Anthomyiidae | 0 | 1 | 0 | 1 | |||
| Empididae | 6 | 3 | 4 | 13 | |||
| Athéricidae | 8 | 0 | 0 | 8 | |||
| Coleoptera | Noteridae | 1 | 0 | 0 | 1 | ||
| Elmidae | 1 | 0 | 0 | 1 | |||
| Dytiscidae | 1 | 0 | 3 | 4 | |||
| Heteroptera | Corixidae | 0 | 0 | 6 | 6 | ||
| Gerridae | 1 | 0 | 9 | 10 | |||
| Crustacea | Decapoda | Atydae (cardina sp) | 2 | 0 | 5 | 7 | |
| Arachnida | Sarcoptiformes | Oribatidae | 4 | 1 | 8 | 13 | |
| Mollusca | Gastropoda | Basommatophora | Planorbidae | 0 | 3 | 1 | 4 |
| Physidae | 0 | 127 | 30 | 157 | |||
| Planorbidae | 0 | 5 | 4 | 9 | |||
| Platyhelminthes | Turbellaria | Tricladida | Dugesiidae | 0 | 158 | 21 | 179 |
| Annelida | Clitellata | Haplotaxida | Naididae | 0 | 29 | 17 | 46 |
| Rhynchobdellida | Glossiphoniidae | 0 | 32 | 4 | 36 | ||
| Nematoda | NI | NI | NI | 0 | 4 | 1 | 5 |
| 196 | 769 | 380 | 1345 |
Table 1. Taxonomic list of macroinvertebrates collected and their total abundances in sampling sites.
The results recorded in this table present the preliminary taxonomic list, as well as the systematic rank of each taxon of the macro-invertebrate communities.
The collected individuals belonged to 5 Phylums, 6 Classes, 15 Orders and 41 Families (Table 1). The analysis of the entire population shows that Arthropods are numerically the most inventoried and represent the highest percentage 67.29% (909 individuals) of the total population. They are abundant at all stations. Platyhelminthes occupy the second position with 13.3% (179 individuals), then Molluscs with 12.64% (170 individuals). Phylums of Annelida and Nematoda are poorly represented. They respectively constitute 6% (82 individuals) and 0.37% (5 individuals) (Fig. 2).
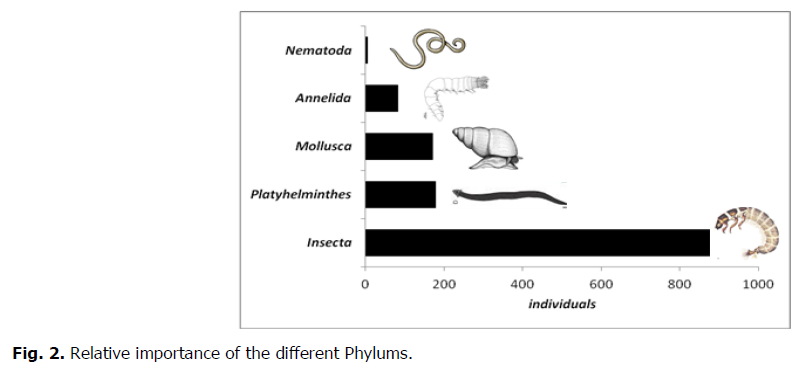
Fig 2. Relative importance of the different Phylums.
In the class of insects which represents almost all of the numbers, Diptera was the most abundant quantitatively Order of the total fauna with more than half of the numbers, they represent 54% of the total abundance, especially in second and the third stations, it was represented by 6 Families; Tipulidae, Chironomidae, Anthomyiidae, Empididae, Simulidae, Athéricidae, while it was present in the first station only. Chironomidae showed the highest numerical abundance which was presented in all stations followed by Simulidae, Tipulidae, Empididae, Athéricidae and Anthomyiidae with one individual.
The Ephemeroptera occupy second place with 31.4% represented by 7 Families, Leptophlebiidae, Baetidae, Hyptageniidae, Isonychiidae, Ameletidae, Oligoneuriidae, Caenidae. This last was the most common Family, with 181 individus followed by Baetidae (82 ind) and other families with a low percentage (Fig. 3).
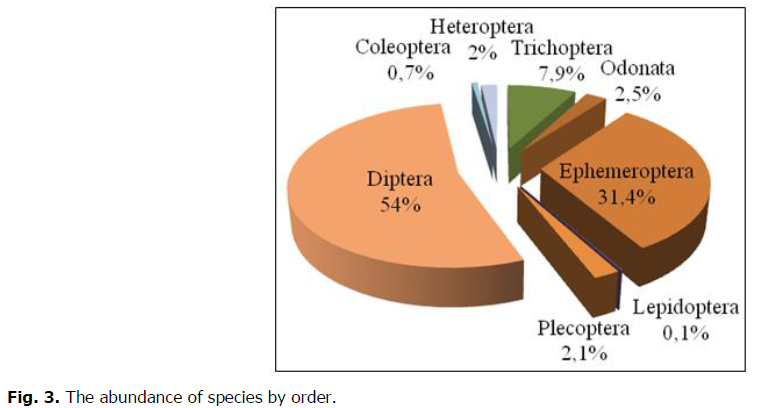
Fig 3. The abundance of species by order.
Trichoptera with 7.9% of total abundance. It was representes by 4 Families; Hydroptilidae, Rhyacophilidae, Leptoceridae and Hydropsychidae which is the most dominant family with 60 individuals.
Two other Order was also represented by 4 Families; Odonata (Libellulidae, Coenagrionidae, Conduliidae, Synthemistidae) and Plecoptera (Perlidae, Leuctrdae, Chloroperlidae, Capniidae) with 2.5% and 2.1% respectively. Heteroptera, Coleoptera and Lepidoptera were the lowest order in term of quantity (less than 2%).
The Families that showed lower abundance were, Leptoceridae, Synthemistidae, Ameletidae, Cambidae, Leuctrdae, Capniidae, Elmidae, Noteridae and Anthomyiidae, each with one individual Sixteen families were recorded at only one site.
Assemblage composition
In order to assess the diversity, different indices have been calculated. The Shannon-Wiener (H') and Simpson's index of diversity, the dominance D, and the Pielou equitability E which provides information on the stability of the environment through the regularity of the population, as well as the Chao-1 estimator which enables to estimate the real specific richness.
The results of the calculations of the various indices are given in the following table.
The stationary taxonomic richness shows fluctuations along the rivers studied. The number of taxa varies from station to station.
The interstation variations of species richness are shown in figure below (Table 2):
| Site1 | Site2 | Site3 | Total samples | |
|---|---|---|---|---|
| Taxa_S | 26 | 19 | 29 | 41 |
| Individuals | 196 | 769 | 380 | 1345 |
| Dominance_D | 0.14 | 0.18 | 0.11 | 0.11 |
| Simpson_1-D | 0.86 | 0.82 | 0.89 | 0.89 |
| Shannon_H | 2.42 | 1.98 | 2.56 | 2.57 |
| Evenness_e^H/S | 0.43 | 0.38 | 0.45 | 0.32 |
| Equitability_J | 0.74 | 0.67 | 0.76 | 0.69 |
| Chao-1 | 48.5 | 24 | 43 | 86 |
Table 2. The different indices calculated for the three stations.
The highest value of Shannon index was recorded in site 3 with 2.56 bits correspond to 29 taxa and 380 individuals with Equitability (E) and Simpson index (1-D) respectively: 0.76, 0.89.
Almost similar values of diversity have been recorded in the site 1 (H: 2.42; J: 0.74). These show that the groups described in this station were well represented.
The lowest diversity and equitability were observed in site 2; (H: 1.98 bits, 1-D: 0.82, J: 0,67). These low values are consequence of the dominance of two families Chironomidae and dugesiidae.
Similarity coefficient
The similarity coefficient or community coefficient makes it possible to characterize, objectively and quantitatively, the degree of resemblance of two lists of taxa. For this purpose, different similarity coefficient formulas have been proposed. Jaccard's coefficients (1901) are among the most commonly used.
That index of Jaccard (1901), which is an asymmetrical coefficient will be used in the present study because the comparisons to be made on the basis of the presence-absence of families and by crossing two different habitats or stations. The results of the Jaccard similarity index calculations are mentioned in the following figure (Fig. 4):
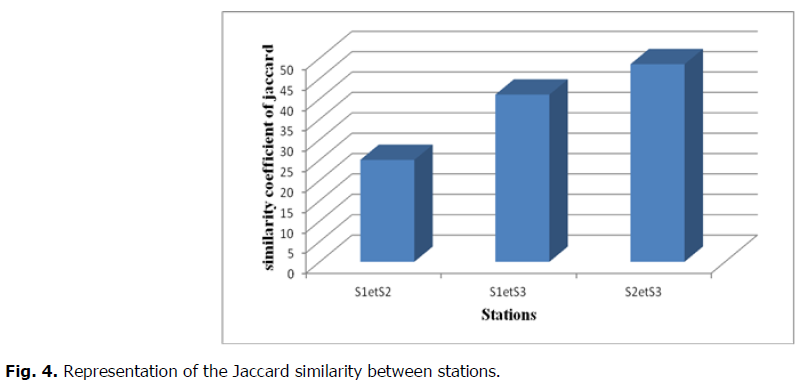
Fig 4. Representation of the Jaccard similarity between stations.
The similarity index is low between stations S1 and S2 (25%) with 35 common families (Figure). The families for the two compared stations are completely different indicating that the different habitat conditions determine a turnover of the important families.
Otherwise, there is a strong similarity crossing the stations "S2 and S3" with 48.48% (32 families) and between the stations "S1 and S3" with 41% (38 families). For each crossing, these stations present almost the same characteristics and even that the similar environmental conditions between the habitats.
Accumulation curves
By accumulating all the samples, the rarefaction curve does not reach asymptote (Fig. 5). Thus, only part of the benthic fauna was sampled. We therefore first evaluated the effect of the protocol sampling on the measurement of species diversity.
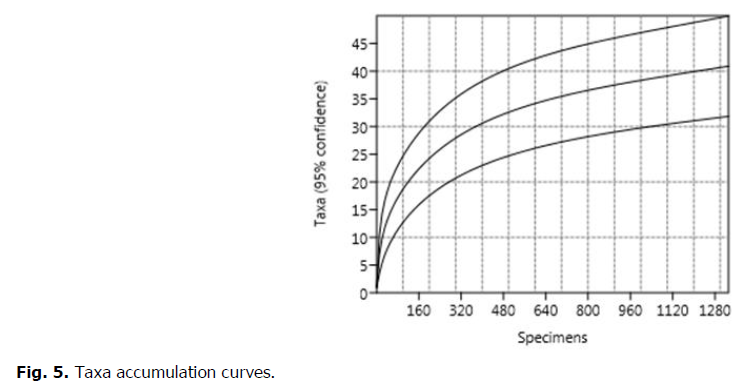
Fig 5. Taxa accumulation curves.
We could say that the difference between the average specific richness per plot and the total richness estimate is acceptable and comes largely from a sampling effort more or less sufficient to determine the species richness within each plot.
Discussion
During our study we found that the assemblages of macro-invertebrates community are different in the because the environemental parameters and type of habitats.
The overall analysis of the benthic fauna of the 3 stations (Oued el Merdja, Oued el Hamdania, Oued Chiffa) made it possible to collect 1345 macro-invertebrates belonging to 41 families and 14 orders. They are collected in the area with an altitude between 400 and 480 m. The number of individuals observed is significantly lower than that found in Oued El Harrach (Bouchelouche, et al., 2013) and in Oued Bouhamdane (Gharbi and Seridi, 2018) and in Oued Chiffa and Mozaia (Arab, et al., 2004) that belong to the same ecological region, but the taxonomic richness (41 taxa) is very close to that found in Oued El Harrach with 40 taxa (Bouchelouche, et al., 2013) and in oued Chiffa with 42 (Arab, et al., 2004). Authors identified 7 orders in the class of insects including Diptera, Ephemeroptera, Trichoptera, Odonata, Heteroptera, Coleoptera, and Plecoptera.
The difference in taxonomic richness that appears in our study would be due to the methodology adopted and the sampling period and the topography, relief and location of the study area.
Most of larval insect were only present in the water in their larval form, while some adult Coleoptera are present in aquatic habitat (Djamai, et al., 2019). Hemiptera are among the most abundant taxa at the end of the hydro period (Karaouzas, et al., 2019, Saoudi, et al., 2018, Florencio, et al., 2009,).
The most abundant organisms was Diptera which are characterized by their great ecological and biogeographical diversity. The first works of this order in Algeria were published by Edwards (1923) and Parrot (1919). Indeed, according to Molineri (2020), the elements of this group of insects have not only a large altitudinal distribution, but also a great capacity to colonize various biotopes, even the most disturbed.
The Ephemeropterans were found with very low densities. They have a high sensitivity to various contaminants, including metals ammoniac and other chemicals (Schletterer, 2010). The low representation of lepidoptera is related to the sampling period (Mohammed, et al., 2021).
These results are consistent with the principle that the diversity index is all the higher when the environmental conditions favor the establishment of new species (Dajoz, 1985, Fisher, 1982).
Indeed, the situation of the river would be favorable to a strong diversity due to the increased food resources of many tributaries located in upstream. On the other hand, such tributaries can be providers of several benthic organisms following the phenomenon of drift (Pelletier, 2002).
Conclusion
Analysis of the structure of the populations of the studied sites shows that the favorable ecological conditions and the great diversity of habitats have allowed the development of a benthic community rich in species and fairly balanced, which shows that the study area is stable since it is part of a protected area that is Chréa National Park. On the other hand, site 2 is the least diversified, due to the negative impacts of the various human activities: construction sites of the north-south highway, to which are added the floods that disturb waterstreams.
The different diversity descriptors used, the specific richness, the Shannon and Simpson index, the dominance and the equitability of the Pielou index, allowed the descriptive study of the stand structure. their values showed a significant level of diversity in the study area.
An overall analysis of the differents results indicates the predominance of organisms tolerant, associated with environmental alterations in the aquatic system, which allow attributing a low quality status for aquatic ecosystems studied. This data reaffirms the importance of using aquatic macroinvertebrates as bioindicators in environmental diagnoses.
The results of this study provide important information and a bridge for further work such as the assemblage-specific responses to environmental disturbance. Our results also contribute to establishing effective management practices, implementing conservation measures, and developing more reliable biological monitoring tools for sustainable freshwater ecosystems.
References
Arab, A., Lek, S., Lounaci, A., Park, Y.S. (2004). Spatial and temporal patterns of benthic invertebrate communities in an intermittent river (North Africa). In Annales de Limnologie-International Journal of Limnology, 40:317-327.
Google Scholar, Crossref, Indexed at
Baaloudj, A., Ouarab, S., Kerfouf, A., Bouriach, M., Hussein, A.A., Hammana, C., Djeneba, H.D. (2020). Use of macro invertebrates to assess the quality of Seybouse River (North-East of Algeria). Ukrainian Journal of Ecology, 10:60-66.
Google Scholar, Crossref, Indexed at
Benzina, I., Bachir, A.S. (2018). Diversity of benthic macroinvertebrates and streams quality in the National Park of Belezma (northern-east, algeria). International Journal of Health and Life Science, 4:1-18.
Google Scholar, Crossref, Indexed at
Bilardo, A., Rocchi, S. (2011). Noteridae, Dytiscidae (Coleoptera) du Gabon (8ème partie). Monts de Cristal. Natural History Sciences, 152:177-231.
Google Scholar, Crossref, Indexed at
Bouchard, R.W. (2004). Guide to aquatic macroinvertebrates of the Upper Midwest. Water Resources Center, University of Minnesota, St. Paul, MN, 208:159-183.
Dajoz, R. (1985). Précis d’écologie. Ed. Bordas.
Djamai, S., Mimeche, F., Bensaci, E., Oliva-Paterna, F.J. (2019). Diversity of macroinvertebrates in Lake Tonga (northeast Algeria). Biharean Biologist, 13:8-11.
Djitli, Y., Boix, D., Milla, A., Marniche, F., Tornero, I., Cunillera-Montcusí, D., Daoudi-Hacini, S. (2021). Annual cycle of water quality and macroinvertebrate composition in Algerian wetlands: A case study of lake Réghaïa (Algeria). Limnetica, 40:399-415.
Dynesius, M., Nilsson, C. (1994). Fragmentation and flow regulation of river systems in the northern third of the world. Science, 266:753-762.
Edwards, F.W. (1923). On some Algerian Species of Simulium. Archive Institute Pasteur d'Algerie.
Everard, M., Powell, A. (2002). Rivers as living systems. Aquatic Conservation: Marine and Freshwater Ecosystems, 12:329-337.
Fisher, S.G., Gray, L.J., Grimm, N.B., Busch, D.E. (1982). Temporal succession in a desert stream ecosystem following flash flooding. Ecological Monographs, 52:93-110.
Google Scholar, Crossref, Indexed at
Florencio, M., Serrano, L., Gómez-Rodríguez, C., Millán, A., Díaz-Paniagua, C. (2010). Inter-and intra-annual variations of macroinvertebrate assemblages are related to the hydroperiod in Mediterranean temporary ponds. Pond Conservation in Europe, pp:323-339.
Google Scholar, Crossref, Indexed at
Ghani, W.M., Rawi, C.S.M., Abd Hamid, S., Al-Shami, S.A. (2016). Efficiency of different sampling tools for aquatic macroinvertebrate collections in Malaysian streams. Tropical Life Sciences Research, 27:115.
Hafiane, M., Hamzaoui, D., Bouchelouche, D., Mebarki, M., Arab, A. (2013). Application de l’IBGN et du BMWP sur un oued temporaire d’Algérie. In USTHB‐FBS‐4th International Congress of the Populations & Animal Communities “Dynamics and Biodiversity of the terrestrial and aquatic ecosystems”" CIPCA4" TAGHIT (Bechar)-Algeria, pp:19-21.
Hamaidi-Chergui, F., Brahim Errahmani, M., Benouaklil, F., Hamaidi, M.S. (2013). Preliminary study on physico-chemical parameters and phytoplankton of Chiffa River (Blida, Algeria). Journal of Ecosystems.
Heidemann, H., Seidenbusch, R. (2002). Larves et exuvies des libellules de France et d'Allemagne (sauf de Corse). Société française d'odonatologie.
Karaouzas, I., Smeti, E., Kalogianni, E., Skoulikidis, N.T. (2019). Ecological status monitoring and assessment in Greek rivers: do macroinvertebrate and diatom indices indicate same responses to anthropogenic pressures?. Ecological Indicators, 101:126-132.
Metzeling, L. (1993). Benthic macroinvertebrate community structure in streams of different salinities. Marine and Freshwater Research, 44:335-351.
Lounaci, A. (2005). Recherches sur la faunistique, l'écologie et la biogéographie des macroinvertebres des cours d'eau de Kabylie (Tizi-Ouzou, Algérie).
Magurran, A.E. (1988). Ecological diversity and its measurement. Princeton University Press.
Meriem, G., El-Houda, S.N. (2018). Initiation à l’identification des macroinvertébrés d’Oued Bouhamdane.
Mimeche, F., Nouidjem, Y., Djamai, S., Alouani, R., Bensaci, E. (2019). Studies on the benthic macroinvertebrate community from K’sob Wadi (M’Sila, Algeria). In International Biodiverstiy and Ecology Sciences Symposium.
Mohammed, Y.M., Arimoro, F.O., Ayanwale, A.V., Adamu, K.M., Keke, U.N., Abubakar, M.D., Achebe, A.C. (2021). The current state of water quality and benthic invertebrate fauna in Chikke Stream (North-Central Nigeria). Ukrainian Journal of Ecology, 11:26-34.
Moisan, J. (2010). Guide d'identification des principaux macroinvertébrés benthiques d'eau douce du Québec, 2010: surveillance volontaire des cours d'eau peu profonds. Développement Durable, Environnement et parcs Québec.
Molineri, C., Tejerina, E.G., Torrejón, S.E., Pero, E.J., Hankel, G.E. (2020). Indicative value of different taxonomic levels of Chironomidae for assessing the water quality. Ecological Indicators, 108:105703.
Muñoz, I., Prat, N. (1994). Macroinvertebrate community in the lower Ebro river (NE Spain). Hydrobiologia, 286:65-78.
Palatov, D.M., Rajabov, Z.P. (2017). Aquatic macroinvertebrates of the Lower Amu Darya. Ukrainian Journal of Ecology, 7:627-632.
Google Scholar, Crossref, Indexed at
Parrot, L. (1949). Quelques notes sur les simulidés d’Algérie. Archives de l’Institut Pasteur d’Algérie, 27:273-276.
Pelletier, L., du Québec, G. (2002). The Saint-Maurice River Basin: Benthic Communities and the Biotic Integrity of the Environment, 1996. Department of Environmental Monitoring, Ministry of the Environment.
Pillar, V.D.P., Orlóci, L. (1996). On randomization testing in vegetation science: multifactor comparisons of relevé groups. Journal of Vegetation Science, 7:585-592.
Reice, S.R., Wohlenberg, M. (1993). Monitoring freshwater benthic macroinvertebrates and benthic processes: measures for assessment of ecosystem health. Chapman and Hall, New York(USA), pp:287-305.
Roy, A.H., Rosemond, A.D., Paul, M.J., Leigh, D.S., Wallace, J.B. (2003). Stream macroinvertebrate response to catchment urbanisation (Georgia, USA). Freshwater Biology, 48:329-346.
Saal, I., Smaoune, G., Bouchelouche, D., Hafiane, M., Hamzaoui, D., Mebarki, M., Arab, A. (2020). Distribution of macroinvertebrates in a river of the Aures (Algeria). Ecosystems, Biodiversity and Eco-Development, 5:79-84.
Saoudi, M., Tadjine, A., Guerfi, S., Necer, A. (2018). A preliminary survey of water physico-chemical characteristics and aquatic macroinvertebrate communities in El Mekhada marsh, north-eastern Algeria. Zoology and Ecology, 28:109-116.
Schletterer, M., Füreder, L., Kuzovlev, V.V., Beketov, M.A. (2010). Testing the coherence of several macroinvertebrate indices and environmental factors in a large lowland river system (Volga River, Russia). Ecological Indicators, 10:1083-1092.
Sellam, N., Zouggache, F., Pinel-Alloul, B., Mimouni, A., Moulaï, R. (2017). Taxa richness and community structure of macroinvertebrates in rivers of different bioclimatic regions of Algeria. Journal of Materials and Environmental Sciences, 8:1574-1588.
Stark, J.D. (1993). Performance of the Macroinvertebrate Community Index: effects of sampling method, sample replication, water depth, current velocity, and substratum on index values. New Zealand Journal of Marine and Freshwater Research, 27:463-478.
Tachet, H., Richoux, P., Bournaud, M., Usseglio-Polatera, P. (2010). Invertébrés d'eau douce: Systématique, biologie, écologie. Paris: CNRS éditions.
Tahar, K.B., Baaloudj, A., Kerfouf, A., Benallal, M.A., Denis, F. (2021). Macrobenthic fauna of the coastal sea beds of Oran's Gulf (Western Algeria). Ukrainian Journal of Ecology, 11:1-4.
Author Info
R. Matallah1*, K. Rabhi2, M. Boumaaza2 and R. El-Farroudji32Department of Natural Science, Faculty of Sciences, University of Medea Yahia Fares, Route nationale N18, Pole Urbain, Médéa (26000), Algeria
3Forest Conservation of Blida, National Park of Chrea, Blida (9000), Algeria
Citation: Matallah, R., Rabhi, K., Boumaaza, M., El-Farroudji, R. (2023). First checklist of benthic macroinvertebrate communities from Chrea National Park, Blida province, Northern Algeria. Ukrainian Journal of Ecology. 13:22-31.
Received: 30-Nov-2022, Manuscript No. UJE-23-88177; , Pre QC No. P-88177; Editor assigned: 02-Dec-2022, Pre QC No. P-88177; Reviewed: 14-Dec-2022, QC No. Q-88177; Revised: 06-Feb-2023, Manuscript No. R-88177; Published: 13-Feb-2023, DOI: 10.15421/2023_426
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
