Research - (2022) Volume 12, Issue 10
Evaluation and study of the physico-chemical, biological (antibacterial and antifungal) characteristics of (Pistacia lentiscus L.) oil originating in three regions of Algeria.
N. Azizi1, N. Hacini1*, H. Sellani2 and K. Selatenia2Abstract
This study consists in a contribution to the valorization of the vegetable oil of the fruit of the lentisque Pistacia lentiscus L. a plant very adapted in the north-east of Algeria. We carried out harvests of the oil traditionally extracted by the rural populations at the levels of the three zones of studies, on which physicochemical analyses (color, moisture, index of acidity, index of peroxide, phosphatide, index of saponification) were realized the physico-chemical parameters of the collected oil were values corresponding to international standards and generally reflecting the good quality of oil, with the exception of the values of acidity index. this physico-chemical study is for the qualitative evaluation taking into consideration its origin (genotype X environment interaction) As the vegetable oil of this plant is a natural cure well known in traditional medicine in Algeria we have performed antimicrobial tests on three strains Pseudomonas, Staphylococcus aureus [Cocci (C+)], Klebsiella pneumoniae [Bacillus (B+)] and antifungal on Verticillium sp, Pythium sp, Phytophthora sp. For the purpose of highlighting the various uses of Pistacia lentiscus. The results reveal the ineffectiveness of all oils against all the bacterial strains tested. On the contrary, the antifungal study shows activities were observed with the oils of the three regions.
Keywords
Pistacia lentiscus L, Antibacterial activity, Antifungal activity, Physicochemical parameters.
Introduction
For millennia, the use of medicinal plants was the main means of healing man. This use is generally adapted to mild pathologies, aiming at symptomatic treatment. There are about 500,000 plants on Earth, about 100,000 of them with medicinal properties, whose active ingredients act directly on the body. They are used in both conventional and herbal medicine. They have advantages that conventional medicines often lack. (Gilles W, 1976; Iserin P, 2001).
The lentiscus (Pistacia lentiscus L.), also known as the lentiscus pistachio, "Derou" or "Tadist," putty tree, is a shrub that grows 1-8 m tall (Iauk et al., 1996), and belongs to the Anacardiaceae (syn. Pistaciaceae) family. The edible fruit of this species is extracted from an oil that was once commonly used for food, lighting, and soap making. In Algeria, oil is traditionally produced in the east, in coastal areas (El-Tarf, Skikda, Guelma, Jijel, etc.), where the species abounds. Traditional medicines practiced on both sides of the Mediterranean attribute lentiscus virtues in the treatment of ulcers, wounds, and light burns. Traditional Algerian medicine uses mainly the fatty oil obtained by the expression of lentiscus fruits in the treatment of small wounds, light burns, and erythema. The oil is also used orally to treat allergic respiratory problems and stomach ulcers.
In Algeria, the Pistachier grows in the wild in both sub-humid and saharan areas (Kadi-Bennane et al. 2005), and its extreme limit is in Hoggar (Monjauze 1980). Recently, AI-Saghir (2010) studying the phylogeny of the genus Pistacia reported that the gene is monophyletic, suggesting two sections: Pistacia and Lentiscella. Several research studies have examined the biochemical composition of Pistacia drupes and its oil (Dansherad and Aynehchi, 1980; Yousfi et al., 2002; Ghalem and Benhsani, 2007; Ghalem and Mohamed, 2009; Saber-Tehrani et al., 2012). Other studies have also been carried out on the antioxidant and pharmaceutical properties of leaves and on the properties of resin extracted from the bark of the pistachier (Somon, 1987; Benhsani et al., 2007).
As a result, the goals of this work are to investigate the physicochemical characterization and biological activities (antibacterial and antifungal) of Pistacia lentiscus oil harvested in three stations in Algeria (El-Tarf, Skikda, and Guelma), in order to validate the oil's effectiveness while taking into account its origin (genotype X environment interaction).
Materials and Methods
Presentation of study areas
Samples of Pistacia lentiscus oil come from three regions in eastern Algeria (Fig. 1), specifically the wilayas of El Taref, Skikda, and Guelma. For the wilaya of El Tarf, the oil has been collected from the commune of Chafia, daïra de Bouteldja (extreme north-east Algerian), characterized by a humid Mediterranean climate and a latitude of 36.611 and longitude of 8.03888 (GPS 36°36′40′′ North, 8°2′20′′ East).
The second collection site is the municipality of Roknia, located 34 km northwest of the wilaya of Guelma (east Algerian), characterized by a semi-arid climate, and a latitude of 36°32'60 and longitude of 7°13'0.01 (GPS 36°32′53′′ North, 7°13′47′′ East). The third site is represented by the municipality of Skikda, located north of the wilaya of Skikda, on the Mediterranean coastline; characterized by a humid climate, its geographical distribution is 36°52′00′′ North and 6°54′00′′ East (Fig. 1).
Fig 1: Location map of the three collection regions (Photo taken from Google Maps).
Plant material
In Algeria, there are four species of Genus Pistacia : Pistacia lentiscus, Pistacia terebinthus, Pistacia vera, and Pistacia atlantica (Quezel P. and Santa S. 1962). Among the species of the genus Pistacia, Pistacia lentiscus L. is a very common shrub in our country (Mitcheh A., 1986, Baudière A et al., 2002). Using random amplified polymorphic DNA (RAPD) analysis, combined with chemical and morphological examinations, a study of the natural variability of Pistacia lentiscus in the Mediterranean basin concluded that there was a high degree of genotypic variability in this species (Barazani O.Z. 2003) (Fig. 2).

Fig 2: Pistacia lentiscus[Anacardiaceae], common shrub of the scrubland and the garigue in Algeria.
Sampling
The samples collected from the vegetable oils of Pistacia lentiscus L., extracted naturally by compression of the material that contained them, previously crushed. Compression can be used cold or hot. were immediately placed in glass bottles, hermetic, wrapped in aluminum foil. A label with the date of collection, the region, and the method of extraction shall be affixed to each vial.
Methods for the evaluation of physico-chemical parameters
This study was carried out at the beautiful plant of Annaba, and the results obtained should make it possible to make a comparative study of these three lentiscus oils (maasra).
The objective of this study is to determine some physico-chemical properties of three samples of lentil oil from three different regions. This is a qualitative study with the purpose of identifying differences between them, influenced by a number of factors (region). The lack of standards for this oil has prompted us to compare our results with those of other vegetable oils such as olive oil.
Chemical analysis
The different physical characteristics of the oil begin with Color is a metric used to assess the amount of pigment responsible for the color of oils. The essential part of the colorimeter (loviband) is 3 series of yellow, red, and blue glasses produced according to the following principle: Each series of glasses is additive, i.e., the absorbance of a glass of a given number is equivalent to the sum of the absorbances of two or more glasses.
The color of the sample placed in a tank with a parallel face is compared with that of the glasses with the aid of a monocular by superimposing the glasses of the yellow, red, and blue series. We seek to obtain the equality of hue of two visible ranges in the monocular; it corresponds to the samples, and the other corresponds to the glasses.
To determine mucilages, take a beaker, put a little oil to test, add a few drops of hydrochloric acid (this acid serves to precipitate mucilages), and heat very strongly until precipitation of mucilages (green or brown flakes) occurs.
Addintionally, The content of water and volatile matter (moisture). by this test we mean by the content of water and volatile matter (moisture) that the loss of mass under the experimental conditions described in the international standards. To determine the humidity of a test sample, we dry it at 103°C+/- 2°C in an oven at atmospheric pressure until a practically constant weight is obtained.
Percentage of water and volatile matter content=(m1-m2) × 100 ÷ T
T: Test sample.
M: Mass in grams of the beaker with the test sample before drying.
M: Mass in grams of the beaker with the test sample after drying.
Unity: Without unity.
Chemical analysis: This is a qualitative study aimed at finding the main parameters. Acidity is the percentage of free fatty acids expressed as a percentage of molecular weight in oleic acid, palmitic acid, or auric acid: 282, 256, 200 g, respectively. The acid number is the number of milligrams of potassium (KOH) required to neutralize the acidity of 1 g of fatty substances.
%Acidity=28.2 × V × N÷P
To find the percentage of oleic acid, it is sufficient to divide the acid number by 2.
%Oleic Acid=Ia÷2
Peroxide index oxidation is a fundamental phenomenon in all fats. The chemical alteration of unsaturated fats by oxygen from the air begins with the formation of peroxide by odometry. It's either the number of micrograms of peroxide per kg of fat or the milliequivalents of active oxygen per kg of fat.
The principles are the oxygenation of potassium iodide and the titration of iodine released by sodium thiosulfate. The peroxide index shall be calculated by the following formula:
IP=(V1-V0)×10/P
The phosphatides
The oil and the phosphors it contains are calcined in the presence of zinc. The colorimetric determination of phosphomolybdate (blue colorimetry) is the most sensitive method, which is particularly suitable for the determination of traces of phosphorus in refined oils.
%phosphatides=(P1 × 100)/P
The saponification index
This is the number of milligrams of KOH required to neutralize the free acidity and saponify the esters of 1 g of lipid. The value of the saponification index allows us to estimate the carbon chain lengths of the fatty acids that make up the oil and to calculate the average molecular weights of the fatty acids and triglycerides that contain the oil.
Statistical analyses
The results were analyzed using R statistical software version 4.0.1 (2020). The data are presented as means standard deviations, and the significance level was set at p 0.05. Analysis of variance (ANOVA one-way) and Tukey's test were performed to determine whether or not there was a difference in physico-chemical parameters in oil samples collected from different regions. Principal
Component analysis (PCR), using a matrix (regions and physicochemical parameters of oils), was also performed to evaluate and classify the oil/physicochemical parameters and the variation between the regions studied.
Evaluation of the antibacterial activity of lentiscus extracts
Origin of the strains The three bacterial strains were isolated from various pathological products from the human microbiology laboratory belonging to the polyclinic MERZOUG IBRAHIM E.P.S.P TARF(ALGERIA) The origin of the samples of the strains is the urine or the pup. The strains to be tested are:
• Gram (-) bacteria: Pseudomonas
• Gram (+) bacteria: Staphylococcus aureus [Cocci (C+)]
Klebsiella pneumoniae [Bacille (B+)]
The microbial strains used are represented by 30 bacterial strains, of which nine are Gram-negative Pseudomonas ATCC 27853 and two are Gram-positive Staphylococcus aureus [Cocci (C+)] ATCC 25923 and Klebsiella pneumoniae [Bacillus (B+)] E47.
The minimum inhibitory concentration (MIC)
It makes it possible to determine the smallest concentration (expressed in micrograms/mL) capable of inhibiting the growth of the bacterium in question (the minimum inhibitory concentration, or MIC). Based on concentration gradients, this is the most commonly used manual method. It is based on the fact that a disk impregnated with antibiotics and "deposited" on a nutrient agar inoculated beforehand by the bacterial suspension to be tested will diffuse according to a concentration gradient and that the bacterium will not develop in the presence of concentrations equal to or greater than the minimum inhibitory concentration.
The process uses disks 6 mm in diameter, the paper of which has been impregnated with an antibiotic of known concentration.
Minimum Bactericidal Concentration (MBC) The lowest antibiotic concentration capable of killing at least 99.99% of the bacteria in an inoculum (<0.01% of survivors) is the value indicative of the bactericidal potency of an antibiotic.
Definition of unit cfu/ml: CFU: Colony Unit
This is a measure of the concentration of Mycoplasma in a sample. It is considered that the concentration is abnormal when it drops from 10 to 5 CFU/mL (10 000 CFU/mL). In our work, the more colonies that appear on our culture agar after sowing the dilute oil, the clearer the microbial suspension is said to be bactericidal (kills all bacteria), and the more cloudy it is said to be bacteriostatic (ash the multiplication of the bacterium) When the MIC is close to the CMB, an antibiotic can be considered bactericidal.
Determination of the minimum inhibitory concentration (MIC) by the dilution method
Preparing the culture medium using a broth water bath or boiling the solid Muller-Hinton culture medium. In front of the benzene beak, the petri dishes are placed, where some milliliters of M.H. medium, already boiled, are emptied. To prepare the strains, the strains stored in the particular media are taken, a few milliliters of nutrient broth (buffered glucosated broth, BGT, or physiological water) are added after stirring the medium, The mixture (nutrient broth+germ) is prepared in petri dishes after being placed on the solid medium Nutrient Agar. And then incubated for 24 hours at 37°C to obtain pure strains for our work.
Preparation of the dilutions of the oils to be tested
In a rack, we put 80 dry tubes with stoppers in which we put solvent. This is the 2nd tube of the rack (we put 1 ml of solvent (D.M.S.O)) Dilution of the oil: In the first tube, we put 1 ml of pure oil (P). In the second tube, we put 1 ml of solvent with 1 ml of pure oil, so we have a dilution of 1/2. In the 3rd tube, 1 ml of the 1/2 dilution is taken and added to 1 ml of solvent, and a 1/4 dilution is obtained. The same handling is done up to the 8th tube, therefore we have decreasing dilutions (1/2.1/4.1/8.1/16.1/32.1/64.1/128) We made dilutions of our oils for the search for the Minimum Inhibition Concentration (MCI). Three sterile disks are added to each tube of the rack (P→1/128) and the following step is taken:
Preparation of the microbial suspension: In a sterile tube and in front of the benzene beak, a few milliliters of physiological water are added. Using a pastor pipette, some colonies of our positive culture are taken from the strains, which are homogenized with the physiological water in the tube.
Preparation of the kneading dish with the M.H. and the microbial suspension: using a swab that has been soaked in our already prepared microbial suspension, it is drained on the wall of the tube, and a culture is made with very tight ridges (seeding) vertical, horizontal, and around the agar.
The disks that have already been soaked in the tubes containing dilutions of the pure at 1/128 are then placed on the M.H. medium that has already been prepared according to the dilutions, and finally, Incube 24 at 48H at 37° C.
Determination of the minimum bactericidal concentration (C.M.B.)
From the microbial suspension previously prepared, a few drops of the latter are added to each dilution tube of the rack, knowing that our dilutions have been divided into two, so we obtain eight dilutions for two rows and take 500 μl of each dilution per meter in another dry tube.In each box of dough, the division is done in four dilutions, so for each oil, two boxes are used → Incubation: 24 hours at 48 hours at 37°C.
Determination of antifungal activity
The antifungal activity of the vegetable oils of Pistacia lentiscus from three regions was carried out at the plant biology laboratory of the University of Chadli Ben Djedid, El-Tarf, in relation to three fungal strains.
Fungal strains The strains used are classified as oomycetes:
1. Sample 06: Verticillium sp.
2. Sample 111: Pythium sp.
3. Sample 120: Phytophthora sp.
We used two methods of work for the first
• Place a very thin layer of culture medium in the kneading boxes.
• Take a small fragment of mushroom and put it in the middle of a kneading dish.
In a rack, place 40 dry tubes with stoppers and add solvent from the rack's second tube
Our oils have been diluted for the Minimum Inhibition Concentration (MIC).
• After 24 hours, the holes are placed in the culture medium, and then the dilutions of the oils prepared are added in these boxes.
• At the end, we put these boxes in the dark at room temperature for 60 hours.
• For the second method, the same steps were followed, except that the culture medium was sterilized with a mixture of antibiotics (2 mL).
Results and Discussion
Physicochemical analyzes
The results presented in Table 1 were obtained by determining the physicochemical parameters.
| Indices | Results | Standards | ||
|---|---|---|---|---|
| Lentisk oil oil from Guelma (El-Roknia) | Lentisk oil from Skikda | Lentisk oilfrom El-Taref (El-Chafia) | ||
| Humidity% | 0.31 | 0.02 | 0.67 | ≤ 1% |
| Acidity% | 8.3 | 3.1 | 7.2 | <3.3% |
| Phosphatide% | 1.171 | 0.319 | 0.567 | 184-196 mg |
| Peroxide value | 5.7 | 3.6 | 1.4 | ≤ 20 meq/Kg |
| colour | ||||
| Yellow | J=22 | J=27.2 | J=33.1 | J ≤ 7 |
| Red | R=4.3 | R=4.3 | R=2.1 | R ≤ 0.8 |
| brown | - | - | B=1.5 | - |
| saponification;index | 163,625 | 165,495 | 167,364 | 188-196 mg |
| Mucilage | Brown | Green | Black | |
Table 1: Physicochemical Characteristics of Three Lentisque Oils (Pistacia lentiscus L.) from: El-Roknia (Guelma), Skikda, and El-Chafia (El-Tarf).
Acidity is the amount of free fatty acids resulting from the hydrolytic reactions of triglycerides. This is a quality criterion for reporting the conservation status of an oil (Kandji, 2001). The high acidity value of our samples (Guelma and El-Tarf) is probably an indication of strong enzymatic hydrolysis of the seeds during the harvest, due to the handling or treatment of oil, according to El Harfi et al. (2015). This high acidity could also be explained by poor preservation of the latter. According to Kandji (2001), when an oil is not subjected to good storage conditions, its quality may deteriorate in various ways, but most often by hydrolysis or oxidation. In this case, it becomes unfit for consumption (Fig. 3).
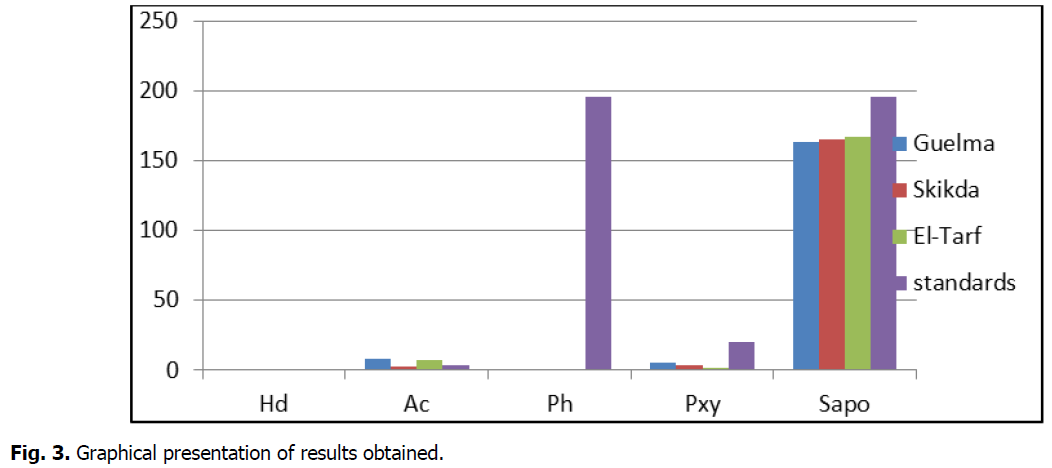
Fig 3: Graphical presentation of results obtained.
Based on the results of Table 1, the moisture content of the three oils is within the standards, so there will be no risk of hydrolysis of saturated fats.
It should also be remembered that all the oils analyzed were extracted in an artisanal manner. Thus, we can produce high acidity by combining the action of additional temperature and water during the process, according to Gholami et al. (2007).
The acidity values obtained for Skikda lentisk oil are 3.1%. This means that the result obtained for lentil oil remains in conformity with the codex standards.
The phosphotide index of lentisk oil from the three regions is within the standards. This is related to the harvesting medium (soil type, climate).
The peroxide index measures the degree of fat rancidity after exposure to air. The latter will lead to the formation of peroxides from unsaturated fatty acids (Kandji, 2001). The oxidation of an oil begins after the fruit is picked from the tree, and continues during storage and processing. Fats can oxidize in the presence of oxygen and certain factors (high temperature, water, enzymes, trace metals: Cu, Fe, etc.). Good hygiene and manufacturing practices will have a positive impact on the peroxide content just after extraction (Chekroun, 2013).
The peroxide index values for the three oils are in accordance with the standards of the Food Codex, which set a value of less than 10 méq of peroxides/kg of oil for a cold-pressed oil. The peroxide index value determined in our study for lentil oil is slightly higher than that found by Boukeloua et al (2012), which is 1.12. This is probably due to the oxidation of the oil studied following the extraction and preservation conditions.
For the color result, lentil oil contains two types of pigments: chlorophylls and carotenoids.
For yellow (Y): this is the chlorophyll criterion, its content varies according to biological and technological factors. And for the color red (R): this is the B-carotene criterion. Based on Table 1, it is noted that the three oils are very rich in colored pigments (chlorophylls and B-carotenes) but this will not pose any health risk to consumers.
The mucilage test showed us the purity of each oil. For the sample of Skikda, the color is "summer green," which conforms to the standards and means that our oil contains only the fruits of the seeds of Pistacia lentiscus L. On the other hand, the other two samples were not extracted under the right conditions, and perhaps there were other substances like the leaves that were mixed with the fruits in these oils.
Interpretation of the results statistically
The "MINITAP16" software was used for PCR and DONDOGRAM to compare the results. The graphical representation in A.C.P. (Fig. 4 and Fig. 5) gives a very good representation of the variables for the two main axes. Representing 59% of the total variance, 38.5% demonstrated the difference between the physico-chemical parameters of the samples and the oil region considered in this study. The second axis captured 20.5% of the total variance and contrasted regions with the highest physicochemical values and parameter indices. In general, the correlation circle shows a greater participation of the "physicochemical parameters" variables
compared to the site variables that explain the G x E interaction.
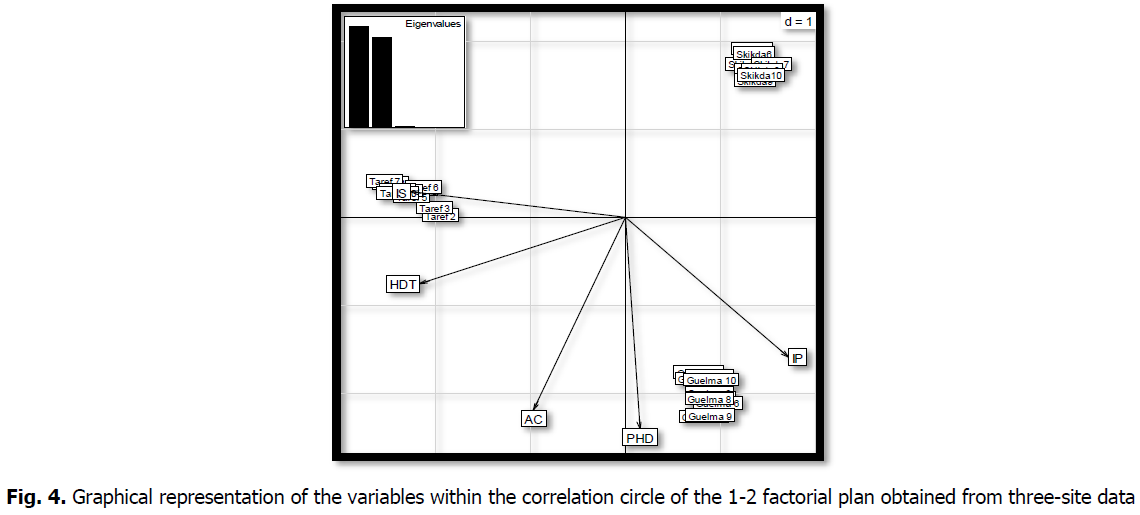
Fig 4: Graphical representation of the variables within the correlation circle of the 1-2 factorial plan obtained from three-site data
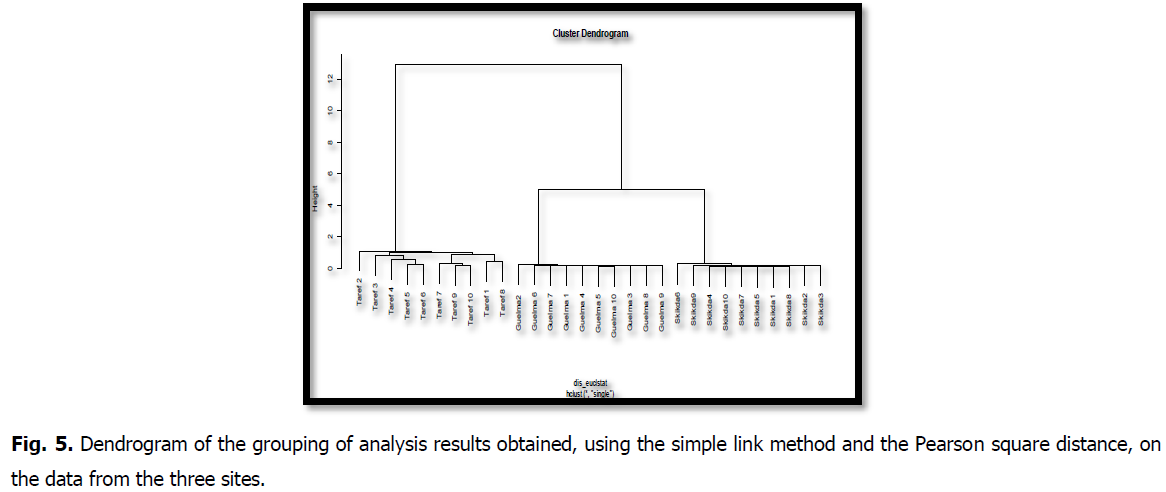
Fig 5: Dendrogram of the grouping of analysis results obtained, using the simple link method and the Pearson square distance, on the data from the three sites.
According to The graphical representations of individuals clearly shows a site effect. The three sites imposed environmental stress on the genotypes, prompting them to respond specifically, as did the differences in soil heterogeneity and agro-climatic data, which differed in terms of meteorological factors.
Results of antibacterial activity
Result of the study of the antibacterial activity of lentil oil
The aromatogram test for lentil oil from the stations studied is shown in Table 2. The reading is done through incubation by marking a zone of inhibition at the periphery of the disk. With these three bacterial strains, our oils have not given any activity (inhibition of bacterial growth), because these latter have specific requirements for antibiotics (Bamou et al., 2015). The results found by (Benroukia and Aouar 2015), which recorded good antibacterial activity with the fruit of Pistacia lentiscus L. on E. coli and S. aureus, are consistent with our results: the oil has antibacterial activity and is absent on others. the results of CMI With these three bacterial strains, our oils have not given any activity (inhibition of bacterial growth), since these latter have specific requirements for antibiotics (Bamou et al., 2015).
| The origin of the oil | bacterial strains Dilution | P | 01-Feb | 01-Apr | 01-Aug | Jan-16 | Jan-32 | Jan-64 | 1/128 |
|---|---|---|---|---|---|---|---|---|---|
| Skikda | Pseudomons | - | - | - | - | - | - | - | - |
| Cocci (C+) | - | - | - | - | - | - | - | - | |
| bacillus (B+) | - | - | - | - | - | - | - | - | |
| Guelma | Pseudomons | - | - | - | - | - | - | - | - |
| Cocci (C+) | - | - | - | - | - | - | - | - | |
| bacillus (B+) | - | - | - | - | - | - | - | - | |
| The oil from d’El-Tarf | Pseudomons | - | - | - | - | - | - | - | - |
| Cocci (C+) | - | - | - | - | - | - | - | - | |
| bacillus (B+) | - | - | - | - | - | - | - | - |
Table 2. Result of antibacterial activity (MIC) of lentiscus oil (Pistacia lentiscus L.) of El-Roknia (Guelma), (Skikda) and (El-Chafia (El-Tarf) on three bacterial strains.
Results of the study of the antifungal activity of lentisk oil (without antibiotics)
With these three strains of fungi, our oils have activity (inhibition of bacterial growth), because Pistacia lentiscus oil contains substances that play an important role in this activity. The MIC in this activity is ½ for all samples (Table 3).
| The origin of the oil | Mushroom strains Dilution | P | 1/2 | 1/4 | 1/8 |
|---|---|---|---|---|---|
| Skikda | Verticillium sp. | ++ | - | - | - |
| Pythium sp. | + | - | - | - | |
| Phytophthora sp. | + | - | - | - | |
| Guelma | Verticillium sp. | + | - | - | - |
| Pythium sp. | + | - | - | - | |
| Phytophthora sp. | + | - | - | - | |
| El-Tarf | Verticillium sp. | + | - | - | - |
| Pythium sp. | + | - | - | - | |
| Phytophthora sp. | + | - | - | - |
Table 3. Shows the antifungal activity (MIC) of lentiscus oil (Pistacia lentiscus L.) from El-Roknia (Guelma) against three fungi strains.
Interpretation of results with antibiotics
Pure oils have an activity (inhibition of bacterial growth) with these three strains of fungi and the presence of antibiotics. Medicinal and aromatic plants, which have been used for centuries to treat human diseases, remain a reliable source of the active principles known by their therapeutic properties.
Conclusion
Pistacia lentiscus L. is an abundant plant species throughout the Mediterranean region, especially in Algeria. Its mature fruits offer us lentisk oil through olive cultivation, whose virtues have been important and known for a very long time.
In Algeria, this olive-growing is mainly practiced in the Eastern Region and remains artisanal, seasonal, and not very profitable. Lentisk oil, which is used primarily as a medicinal product due to its high level of active molecules, is sold at high prices. As a result of the progressive exploitation of this species and the growing interest of Algerian families, lentil oil has become a topical subject in the sphere of scientific research.
This work focuses on the characterization of this little-known oil, collected from different regions of eastern Algeria (El Tarf, Guelma, and Skikda) and extracted according to different artisanal methods, by assaying its physico-chemical parameters and then evaluating the antibacterial and antifungal activity.
Our results show that the geographical origin (altitude, climate, soil nature, etc.) and the extraction method have a direct influence on some physico-chemical parameters and may have consequences on the indices of acid, peroxide, composition of fatty acids and chlorophylls, and the moisture of the oil. Despite this, and as an example, there are differences between the samples in terms of acidity values (8.3% for the Guelma sample, followed by 7.2% for the Chafia sample, after 3.1% for Skikda).
The peroxide index values are 5.7 meq O2/kg for lentisk oil from the Guelma region, 3.6 meq O2/kg for lentisk oil oil from the Skikda region, and 1.46 méq O2/kg for El Tarf.
These values are in accordance with the standards of the Codex Alimentarius, which has set a value of less than 10 méq of peroxides/kg oil for a cold-pressed oil. The highest saponification value corresponds to the oil sample from the El-Tarf region (167.364).
The value of the saponification index of the two samples of lentisk oils is much more in agreement with values reported for other oils. Karleskind, A. (1992). In addition, it should be noted that, even though their values vary from one sample to another, the oil remains good for consumption.
The antibacterial evaluation showed that P. lentiscus vegetable oil in the three regions had no activity against the strains tested, Basilus klibcialla Gram (+) and Staphylococcus aureus Gram (-). Our results are consistent with those found by Meddour et al., (2013), which showed that the optimal efficacy of an extract may not be due to a main active component, but to the combined action (synergy) of different compounds composed of the origin of this extract (Essawi and Srour, 2000). Thus, unlike fractionated extracts and essential oil, crude extracts are the only ones to have shown an inhibitory effect on the different bacterial strains.
Besides, herbal medicine recommends using the whole plant, also called "Totum," rather than laboratory extracts (Iserin et al., 2001).
Results of fungal activity show that vegetable oils exert a synergistic effect against fungal strains at a specific concentration. As a result, we can conclude that our oils have significant antifungal activity on the mushrooms tested…The antifungal performance demonstrated warrants an additional study in order to consider the prospects of application of these active principles as control and bio-conservation agents capable of reducing the growth of fungi and molds.
Our study confirms the value of lentil oil, thus offering a heritage to preserve, develop, and enhance, since our results, together with those of previous studies, constitute an essential basis for their exploitation in the appropriate fields.
This study once again makes it possible to highlight the exploitation of essential oils in the pharmaceutical and medical fields.
References
AFNOR. (1984). Détermination de l’Indice de réfraction. Edition AFNOR, Paris.
AFNOR. (1988). Corps gras, graines oléagineuses, produits dérivés. 4th Edition, AFNOR, Paris, p:531.
AL-Saghir, M.G. (2010). Phylogenetic analysis of the genus Pistacia L.(Anacardiaceae) based on morphological data. Asian Journal of Plant Sciences, 9:28.
Atmani, D., Chaher, N., Berboucha, M., Ayouni, K., Lounis, H., Boudaoud, H., Atmani, D. (2009). Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chemistry, 112:303-309.
Baba Aissa, F. (1999). Encyclopédie des plantes utiles. Flore d’Algérie et du Maghreb. Substances Végétales d’Afrique, d’Orient et d’Occident", Librairie Moderne Rouiba, EDAS, Alger.
Barazani, O., Dudai, N., Golan-Goldhirsh, A. (2003). Comparison of Mediterranean Pistacia lentiscus genotypes by random amplified polymorphic DNA, chemical, and morphological analyses. Journal of Chemical Ecology, 29:1939-1952.
Bammou, M., Daoudi, A., Slimani, I., Najem, M., Bouiamrine, E.H., Ibijbijen, J., Nassiri, L. (2015). Valorisation du lentisque «Pistacia lentiscus L.»: Étude ethnobotanique, Screening phytochimique et pouvoir antibactérien. Journal of Applied Biosciences, 86:7966-7975.
Baudière, A., Monange, Y., Gauquelin, Th. (2002). Le Monde des Plantes; Intermédiaire des Botanistes, Toulouse; 477:2-5.
Belhadj, S. (1999). Les pistacheraies algériennes: Etat actuel et dégradation. Cahiers Options MED, 56:107-109.
Benhassaini, H., Bendahmane, M., Benchalgo, N. (2007). The chemical composition of fruits of Pistacia atlantica desf. subsp. atlantica from Algeria. Chemistry of Natural Compounds, 43:121-124.
Benhassaini, H., Mehdadi, Z., Hamel, L., Belkhodja, M. (2007). Phytoécologie de Pistacia atlantica Desf. subsp. atlantica dans le Nord-Ouest algérien. Science et Changements Planétaires/Sécheresse, 18:199-205.
Benrokia, H., Anouar, K. (2015). Etude de l’activité Antibactérienne des extraits de Pistacia lentiscus.
Boukeloua, A., Belkhiri, A., Djerrou, Z., Bahri, L., Boulebda, N., Pacha, Y.H. (2012). Acute toxicity of Opuntia ficus indica and Pistacia lentiscus seed oils in mice. African Journal of Traditional, Complementary and Alternative Medicines, 9:607-611.
Chekroun, N. (2013). Détermination de la capacité antioxydante des huilesvégétales: Huile Afia. Mémoire de fin d’étude. Université Abou Bekr Belkaid. Tlemcen, p:72.
Chiej, R. (1982). Les Plantes Medicinales, édition Solar, Paris, France, p:235.
Daneshrad, A., Aynehchi, Y. (1980). Chemical studies of the oil from pistacia nuts growing wild in Iran. Journal of the American Oil Chemists' Society, 57:248-249.
Bachir, R.G., Benali, M. (2009). Bactericidal activity of Pistacia atlantica. Desf mastic gum against certain pathogens. African Journal of Plant Science, 3 :013-015.
Ghalem, B., Benhassaini, H. (2007). Etude des phytostérols et des acides gras de Pistachia atlantica. Afrique Science: Revue Internationale des Sciences et Technologie.
Gilles, W. (1976). L’encyclopédie des médecines naturelles et des secrets de santé. Elina, Lavoisier, Paris, pp:212-222.
Iauk, L., Ragusa, S., Rapisarda, A., Franco, S., Nicolosi, V.M. (1996). In vitro antimicrobial activity of Pistacia lentiscus L. extracts: preliminary report. Journal of Chemotherapy, 8:207-209.
Iserin, P., Masson, M., Restellini, J., Ybert, E., De Laage de Meux, A., Moulard, F., Vican, P. (2001). Larousse des plantes médicinales: identification. Préparation, soins. 2nd Edition Larousse, VUEF, pp:13-16.
Kadi-Bennane, S., Ait-Said, S., Smail-Saadoun, N. (2005). Etude adaptative de trois populations de Pistacia atlantica Desf. ssp. atlantica (Ain Oussera-Messaad-Taissa) par le biais du complexe stomatique. Options Méditerraneennes, 63:365-368.
Kandji, N.A. (2001). Etude de la composition chimique et de la qualité d’huiles végétales artisanales consommées au Sénégal. Tours, France Thèse de Pharmacie.
Karleskind, A. (1992). Manuel des corps gras.
Mitcheh, A. (1986). Tous les Arbres de nos Forêts, édition Bordas, p:319.
Monjauze, A. (1980). Connaissance du bétoum Pistacia atlantica Desf. Revue Forestière Française, 32:356-363.
Saber-Tehrani, M., Givianrad, M.H., Aberoomand-Azar, P., Waqif-Husain, S., Jafari Mohammadi, S.A. (2013). Chemical composition of Iran's pistacia atlantica cold-pressed oil. Journal of Chemistry.
Quézel, P., Santa, S. (1962). Nouvelle flore de l'Algérie et des régions désertiques méridionales.
Somov, E. (1987). Arbres, arbustes et arbrisseaux en Algérie.
Yousfi, M., Nedjmi, B., Bellal, R., Bertal, D.B., Palla, G. (2002). Fatty acids and sterols of Pistacia atlantica fruit oil. Journal of the American Oil Chemists' Society, 10:1049-1050.
Author Info
N. Azizi1, N. Hacini1*, H. Sellani2 and K. Selatenia22Department of Biology, Faculty of Nature and Life Sciences, Chadli Bendjedid University of El Tarf, Algeria
Citation: Azizi, N., Hacini, N., Sellani, H., Selatenia, K. (2022). Evaluation and study of the physico-chemical, biological (antibacterial and antifungal) characteristics of (Pistacia lentiscus L.) oil originating in three regions of Algeria. Ukrainian Journal of Ecology. 12:34-44.
Received: 23-Oct-2022, Manuscript No. UJE-22-78082; , Pre QC No. P-78082; Editor assigned: 25-Oct-2022, Pre QC No. P-78082; Reviewed: 05-Nov-2022, QC No. Q-78082; Revised: 10-Nov-2022, Manuscript No. R-78082; Published: 15-Nov-2022, DOI: 10.15421/2022_406
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
