Research - (2021) Volume 11, Issue 3
Effect of water stress on the density of rhizospheric fungi, Actinomycetes and Azotobacters associated with cereal cultivation
H.Marouane1, K. Oulbachir1 and K. Acem2*Abstract
The study evaluates the effect of water stress on the microorganisms biomass of the rhizosphere associated with cereal cultivation. The latter selects the most resistant germs to introduce them into the improvement of the system: soil-plant under water stress condition. The study of the effect of water deficit on the rhizospheric microbial biomass under a cereal crop (durum wheat) in situ reveals a reduction in the density of Actinomycetes, Azotobacters, and fungi. First, concerning the cultivated soil subjected to rainfall, the number of Actinomycetes gradually decreases from 25.5.106 germs/g to 3.33.106 germs/g. Whereas the cultivated soil irrigated at 50% to the field capacity, their number also decreases from 43.106 germs/g to 3.9.106 germs/g. The cultivated soil is irrigated at 100% to its field capacity; the density gradually decreases from 210.106 germs/g to 5.76.106 germs/g. Similarly, in the cultivated soil subjected to rainfall, the Azotobacters rate gradually decreases from 36 .106 germs/g to 10.7.106 germs/g. Regarding the cultivated soil irrigated at 50% to field capacity, the density gradually decreases from 91.106 germs/g to 5.03.106 germs/g. In the cultivated soil that was 100% irrigated to the field capacity, the density gradually decreases from 1.3.106 germs/g to 2.106 germs/g. This reduction is achieved under humidity values close to the wilting point of loamy, sandy soil. The highest values were recorded at the vegetative stage: tillering. The study revealed that the effect of water stress on the evolution of fungi is less significant. There is a correlation between their density and some pedoclimatic parameters, which vary under water stress, such as the pH of the studied soil. It has been recorded that Actinomycetes were the least sensitive to water stress, unlike Azotobacters. Hence, they can be the most valuable microorganisms as auxiliaries for improving plant production, namely PGPR (Plant Growth Promoting Rhizobacteria).
Keywords
Water deficit, microbial biomass, rhizosphere, durum wheat, Actinomycetes, fungi, Azotobacters, irrigation
Introduction
The biomass is the living matter quantity present in the soil; its stability results from a dynamic equilibrium. At any time, organisms are born, multiply and die. The microorganisms degrade the substrate, synthesize the new organic matter, and then, in turn, lysed. Their total number varies depending on environmental conditions and all external interventions (Davet, 1996). The qualitative and quantitative composition of biomass is one of the characteristics that help define a soil; moreover, this composition results in part from the physicochemical nature and structure of the soil. The maintenance of the biomass is ensured by the nutritional contributions coming from the vegetation (Carnavalet, 2015).
Plant productivity depends on the climate, and the richness of the soil with elements that the plant can assimilate. Therefore, it is partly conditioned by the adequate recycling of the organic matter: an ecosystem, natural or cultivated, only functions normally if microbial life is active (Davet, 1996). Nowadays, another problem is posed, threatening the whole world: Global warming, which induces drought by causing water stress, which attacks plant production (Lepoivre, 2003). One billion hectares are threatened worldwide, including 3.2 million in Algeria (Belkhodja & Bidai 2004). The irregularity of the drought impacts plants that depend on its intensity and the time of its onset (Lopez et al., 2003). The cereal region of Algeria is located mainly in the interior plains and high plateaus, where the constant water shortage significantly limits the productive potential of the main cultivated grains. Among the latter, durum wheat dominated the crops for several reasons (Adda et al., 2013).
Plant Growth Promoting Bacteria (PGPR) can improve the performance and resistance of plants under environmental stress. Under natural conditions, many parameters can influence the adaptive capacities of plants in the face of abiotic stresses. Among these, the physicochemical quality of the soil or the presence of microorganisms that can act on the availability of resources or the functioning of plants (Bresson, 2013). Agricultural practices are changing, heavy reliance on non-renewable inputs and intensification based on chemicals, such as pesticides, are gradually giving space to other forms of intensification based on natural biological processes and biodiversity to increase agricultural ecosystems (FAO, 2011).
Accordingly, various interactions in the rhizosphere exist between the soil, the plant, and the microorganisms (Gobat, 2010). This study evaluates the effect of water stress on the biomass of microorganisms in the rhizosphere associated with cereal cultivation. Such evaluation helps select the most stress-resistant microorganisms for using them to improve the soil and the plant under stress conditions (Carnavalet, 2015).
Materials and Methods
Experimental site
The experiment was carried out at the "Technical Institute of Great Cultures" of Dahmouni, Wilaya of Tiaret, which is part of the high cereal plains of western Algeria.The climate is the semi-arid Mediterranean with a mean annual temperature of around 15.4°C and an annual rainfall of 410 mm. Water stress in this area is one of the major problems that minimize plant production (Benouadah et al., 2020).
The studied cereal crop is the durum wheat crop composed of two varieties; Vitron (V1) and Bousselem (V2).
Water stress
From the emergence stage to the filling stage to the end of the crop, the effect of the water stress is determined by a comparative study between an irrigated crop at field capacity (50%, 75%, and 100%) and a crop of the same varieties subjected to rainfall. Water stress in the studied area is one of the significant problems that minimize plant production. In this study, the water stress sets naturally during the vegetative stages of bolting and filling are confirmed by the drop in yield parameters (Gallais, 1992) and (Carnavalet, 2015).
The experimental device
The experimental device adopted is the randomized complete block, composed of four blocks; each one contains three plots, each subjected to four nitrogen treatments; T1, T2, T3, and T4. These blocks are classified as follows: (Fig. 1).
- A pluvial block subjected to rainfall.
- An irrigated block at 50% of the field capacity.
- An irrigated block at 75% of the field capacity.
- An irrigated block at 100% of the field capacity.

Figure 1: Diagram of the experimental device.
Soil
The soil was taken from the surface horizon (0-20 cm), dried, crushed, and then sieved at 2 mm (Clément, 1998) (Baize, 2018). During each vegetative stage, a sample is taken randomly from each plot of the adopted experimental device.
Soil analyses:
Granulometry
It was determined by the international Robinson's pipette method, which consists of repeatedly taking samples of the suspension at a fixed height (Baize, 2000).
Physico-chemical parameters of the soil:
The physicochemical analyses were carried out according to (Baize, 2000, and 2018)
Humidity
Soil moisture was determined by weight loss after drying at 105 ° C (Baize, 2000).
pH water:
The pH was measured by a HANNA type pH meter.
Total limestone (total carbonates:
Most often, this value is determined by a “volumetric calcimeter”.
Electrical conductivity:
It was measured by the conductometric method.
Carbon and organic matter:
The organic carbon content was determined by the Anne method.
Microbiological study of the soil:
The method for evaluating Actinomycetes, Azotobacters, and fungi: Indirect counting in a solid medium: The principle is to spread the agar nutrient medium on the surface of the boxes with a measured quantity of suspension-dilutions of soil. After that, the count of the colonies, which appeared after seven days of incubation, would be conducted (Calvet, 2003) and (Prescott, 2018).
Data analysis
The obtained results were subjected to analysis of variance (ANOVA) at a probability level of 5%.
Results
Granulometry
The studied soil has a sandy loam texture. This texture is confirmed by several studies carried out in the same region. The area of this study is recognized by the high rate of silt (Oulbachir, 2010); this texture determines soil properties, related to the water state (Gobat, 2010) (Table 1).
Table 1. Water properties of the studied soil.
| The apparent density g/cm3 | Moisture to the field capacity (%) | Moisture to the field capacity (mm) | Moisture at the wilting point (%) | Moisture at the wilting point (mm) | The valuable reserve UR (mm) |
|---|---|---|---|---|---|
| 1.32 | 19 | 50.16 | 9.66 | 25.5 | 24.7 |
Soil moisture
The statistical analysis shows that water stress affects soil moisture. This effect is confirmed by the comparative study between irrigated and non-irrigated crops subjected to water stress conditions (Table 2).
Table 2. Soil moisture level at the start of the study.
| Bare ground | Non-irrigated cultivated soil | Cultivated soil irrigated at 50% to the field capacity | Cultivated soil irrigated at 100% to the field capacity | ||||
|---|---|---|---|---|---|---|---|
| Soil moisture rate (%) | 10.33 | 9.33 | 8.33 | 8.33 | |||
As soon as water stress sets in, it is noticed that it significantly affects soil moisture (p = 0.032). The humidity gradually decreases from the start of the crop cycle until the end of the agricultural cycle, and the results are as follows:
The humidity of the soil where the crop is subjected to climatic conditions is 10% and then decreases to 5.44%; this value increases after a low rainfall synchronized with the end of the studied cereal cultivation at 6.8%.
The humidity of the soil where the crop is irrigated at 50% of the field capacity is 20.333% which decreases to 10.89%; this value decreases to 8.43% at the end of the studied cereal cultivation.
The soil moisture where the crop is irrigated at 100% of the field capacity is 30.33 3% which decreases to 15.01% and decreases to 6.2% at the end of the cultivation.
Physico - chemical parameters of the soil
Water stress affects the physicochemical parameters of the studied soil. This impact differs from one parameter to another. These parameters are related to each other and the density of soil microorganisms (Baize, 2018). The statistical study by correlation matrix shows a negative correlation between the humidity rate, the pH, and the total limestone. The total limestone rate is positively correlated with the pH and the electrical conductivity. The correlation of the PH is negative with organic matter. The pH has a negative correlation with the electrical conductivity and density of fungi. Soil microorganisms have a positive correlation with soil moisture level and organic matter level. It should be noted that the physicochemical parameters of the soil vary with the variation of the vegetative stages (the emergence stage, tillering, stemming/heading, and filling). The results are presented in Table 3 (Fig. 2).
Table 3. Correlation matrix between soil parameters and soil microorganisms under water stress.
| Humidity % | Total limestone % | pH | Electrical conductivity µS /cm | Organic material % | Azotobaters germ/g de sol | Actinomycetes germ/g de sol | Fungi germ/g de sol | |
|---|---|---|---|---|---|---|---|---|
| Humidity % | 1 | |||||||
| Total limestone % | -0.153368113 | 1 | ||||||
| pH | -0.277978742 | 0.653855226 | 1 | |||||
| Electrical conductivity µS/cm | 0.03200541 | 0.288334018 | 0.528549122 | 1 | ||||
| Organic material % | 0.315313416 | -0.602980268 | -0.867889989 | -0.548709913 | 1 | |||
| Azotobaters germs/ g de sol | 0.483279569 | 0.07041373 | 0.168183148 | -0.005772979 | 7.20115E-06 | 1 | ||
| Actinomycetes germs/ g de sol | 0.738117445 | 0.044652368 | 0.183477973 | 0.042506617 | 0.027649321 | 0.645795453 | 1 | |
| Fungi germs/ g de sol | 0.0042463 | -0.332362944 | -0.352544112 | -179856915 | 0.451264597 | -0.149827048 | -0.1213433 | 1 |
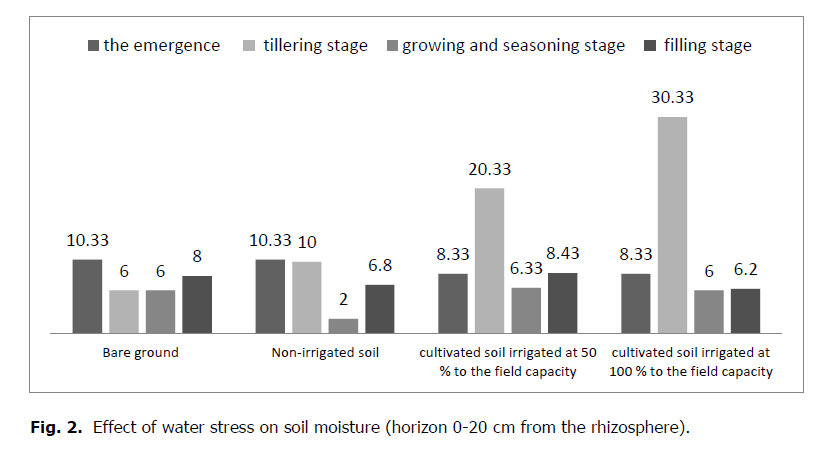
Figure 2: Effect of water stress on soil moisture (horizon 0-20 cm from the rhizosphere).
Value> 1 no correlation, value <1 There is a correlation, (+) the correlation is positive, (-) the correlation is negative.
Effect of water stress on the density of soil microorganisms
The soil moisture affected by water stress has a significant effect on Actinomycetes (p = 0.01). For the bare soil, their number gradually decreases by 10.6 .106 germs/g of soil to 9.25.106 germs/g. Concerning the soil cultivated under rainfall, the density gradually decreases from 25.5.106 germs/g to 3.33.106 germs/g. Concerning the cultivated soil irrigated at 50% to the field's capacity, the density decreases from 43.106 germs/g to 3.9.106 germs/g. While the cultivated soil 100% irrigated at field capacity, the number also decreases, from 210.106 germs/g to 5.76.106 germs/g.
Moreover, the number of Actinomycetes in bare soil is less than the number of cultivated soil. Thus, the number of the irrigated soil is more significant than non-irrigated soil. The highest density is reported during the vegetative tillering stage.
It has been noticed that the humidity of the soil affected by water stress has a highly significant effect on Azotobacters (p = 0.0007). Regarding the bare soil, Azotobacters number gradually decreases by 18.6 .106 germs/ g of soil up to 2.2.106 germs/g. The cultivated soil subjected to rainfall, their number gradually decreases from 36.106 germs/g to 10.7.106 germs/g. Regarding the cultivated soil irrigated at 50% of the field's capacity, the density also gradually decreases from 91.106 germs/g to 5.03.106 germs/g. The cultivated soil is irrigated at 100% of the field's capacity; their number also decreases gradually from 5 1.3.106 germs/g up to 2.106 germs/g (Tables 4 and 5).
Table 4. Result of the ANOVA analysis demonstrating the effect of humidity on Actinomycetes density.
| Groups | Number of sample | Sum | Average | Variance | |||||||
| Humidity % | 16 | 1,537,666,664 | 9,610,416,652 | 4,534,336,562 | |||||||
| Actinomycetes germ/g | 16 | 561840000 | 35115000 | 2.65E+20 | |||||||
| Variance analysis: | |||||||||||
| Critical value for F | Source of variations | sum of squares | Degree of liberty | Average of squares | F | Probability | |||||
| 4.170876757 | Between Groups | 9.8645E+15 | 1 | 9.8645E+15 | 7.438960895 | 0.01056143 | |||||
| Within groups | 3.97818E+16 | 30 | 1.32606E+15 | ||||||||
| Total | 4.96463E+16 | 31 | |||||||||
Table 5. Result of the ANOVA analysis demonstrating the effect of humidity on Azotobacters density.
| Groups | Number of samples | Sum | Average | Variance | |||||||
| Humidity % | 16 | 153.7666664 | 9.610416652 | 45.34336562 | |||||||
| Azotobacters germ/g | 16 | 364030000 | 22751875 | 5.91674E+14 | |||||||
| Variance analysis: | |||||||||||
| Source of variations | Sum of squares | Degree of liberty | Average of squares | F | Probability | Critical value for f | |||||
| Between groups | 4.14118e+15 | 1 | 4.14118e+15 | 13.99817209 | 0.000773085 | 4.170876757 | |||||
| Within groups | 8.87511e+15 | 30 | 2.95837e+14 | ||||||||
| Total | 1.30163e+16 | 31 | |||||||||
The number of Azotobacters in bare soil is lower than the cultivated soil number. The number in the irrigated soil is more than the number of Azotobacters in non-irrigated soil. After the humectation, the number of Azotobacters increases slightly. The highest number of Azotobacters is reported during the tillering stage (Fig. 3 and 4).
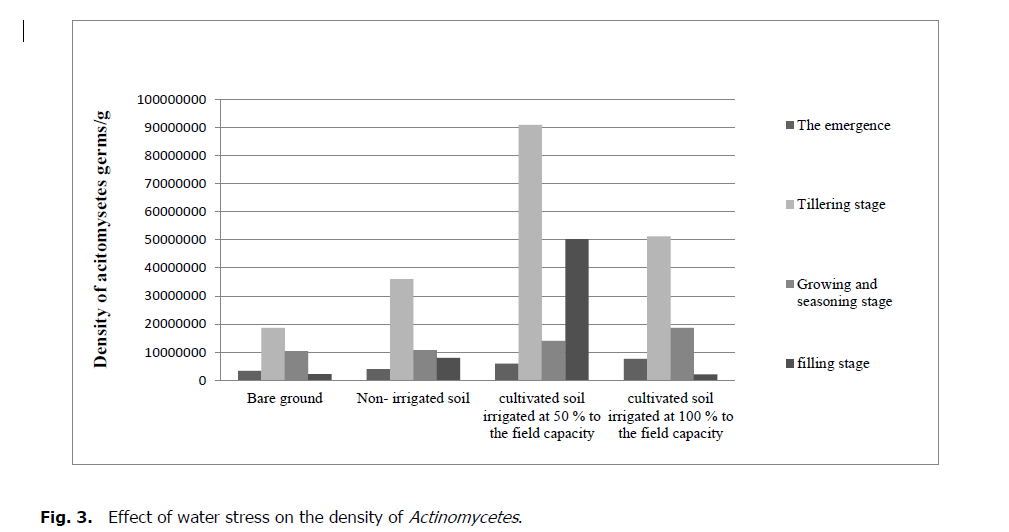
Figure 3: Effect of water stress on the density of Actinomycetes.
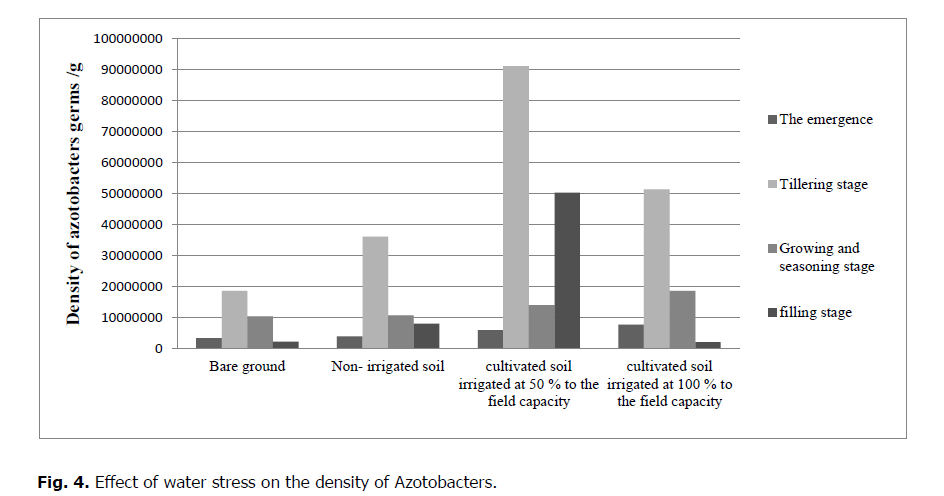
Figure 4: Effect of water stress on the density of Azotobacters.
The effect of water stress on the density of fungi is small. However, the variation in their number (fungi) is correlated with other soil parameters affected by the water stress, such as pH, which is negatively correlated with these microorganisms (Fig. 5).
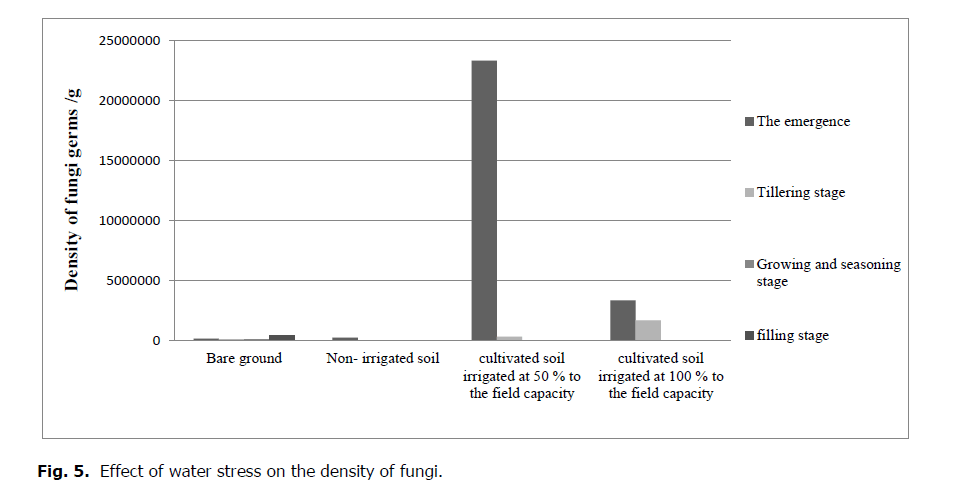
Figure 5: Effect of water stress on the density of fungi.
Discussion
According to the obtained results, the study of the effect of water stress (deficit) on the rhizospheric microbial biomass of the cereal cultivation showed that the microbial biomass is sensitive to water stress indirectly. This was confirmed by the results and the statistical study, where it was noticed that soil moisture affected by water stress has a significant impact on Azotobacters and Actinomycetes. Hence, the density gradually decreases with decreasing the soil moisture induced by the stress (Calvet, 2003). Similarly, the number of Azotobacters and Actinomycetes increases with irrigation and decreases in its absence (the stress).
Intense desiccation always results in the death of large numbers of creatures. The intensity of this phenomenon increases with the amount of water that can be accessed, the duration of the drought period, and the temperature. The survival rate of microorganisms depends mainly on their physiological state. The youngest and most active fraction of the biomass, which was in the exponential phase of growth at the time of dehydration, is the most vulnerable. The fraction produced in the stationary or dormant state is, on the contrary, affected slightly (Davet, 1996). The water regime of the soil depends directly on the properties of the soil, such as the texture, which determines the forces of water retention, the structure, and the porosity that define the volume of the water reservoir of the soil (Gobat, 2010). On the other hand, the impact of stress on fungi is less significant. According to (Calvet, 2003); the water content of the soil influences the activity of the microflora through its relationship to both the bioavailability of water and the aeration of the soil.
The recorded results also show that Actinomycetes are the least sensitive microorganisms to water stress, while Azotobacters are more sensitive to the decrease in humidity due to the water stress. These results are confirmed by (Davet, 1996), who mentioned that Actinomycetes and cyanobacteria are more resistant than average. Azotobacters, on the contrary, are very sensitive. Gram-negative bacteria, with thin walls, are generally less resistant than Gram-positive bacteria; this may explain the ability of Actinomycetes to resist stress because they are Gram-positive bacteria. According to Davet (1996) Bacteria are, on average, more sensitive than fungi. Their activity is low around the wilting point (-1.5 MPa), stops around - 6 to - 8 MPa, but there are substantial variations among taxonomic groupings.
In this study, it was noticed that the fungi are sensitive and affected by the decrease of water. Likewise, the number of Actinomycetes is negatively correlated with fungi, where Actinomycetes are linked to the absence of fungi. This was explained by the effect of Actinomycetes, which are known for their power to synthesize antibiotics and which have an essential antagonistic role against fungi (Davet, 1997).
The number of fungi is negatively correlated with the variation of the soil pH, which changes from neutral to alkaline. The acidic environments are the most favorable for the fungi. Alkalinity has a negative effect on fungi. However, pH can be considered to influence the microbial composition of the soil and specific aspects of the activity of microorganisms (Davet, 1997). It also plays a role in adhesion to clay particles (effect on the variation of surface electrical charges, microbial walls, absorption of minerals, and natural attraction between bacteria or conidia and clay particles). Fungi are generally predominant in acidic soils, while bacteria predominate in neutral or slightly alkaline soils (Davet, 1997). The change in pH indeed leads to multiple consequences, such as metabolite synthesis only taking place within very narrow pH limits, sporulation. Actinomycetes, which have an essential antagonistic role against fungi, are particularly sensitive to acidity. It is noticed that the variation of the pH values during this study changes from neutral 7.01 to alkaline pH values up to 7.8. Soil microorganisms can modify the pH through their action on mineral or organic compounds. Also, they can the biological oxidation of ammoniacal salts resulting in the formation of nitrates. By synthesizing organic acid, a wide variety of organic acids appears during the mineralization of organic matter. Some fungi have a very high acidifying power (Davet, 1997). The alkalinization of the medium may be due to the hydrolysis of proteins and nitrogenous organic compounds in general (urea). The latter leads to the formation of ammonia, which raises the ambient pH.
The studied cultivation subjected to climatic conditions where there was a light rainfall at the end of the agriculture cycle caused an increase in certain microorganisms. The rewetting of dry soil causes a rapid resumption of activity, which for a few days is greater than the microbial activity in a sample of the same soil kept constantly moist (Gobat, 2010). Also, water stress has a significant effect on the productivity of the studied crop. It causes a drop in the yield confirmed by the variations in yield parameters between an irrigated and non-irrigated crop (Gallais, 2015).
It was noted that there is a relationship between the evolution of the vegetative stages of the studied durum wheat cultivation (emergence, tillering, bolting, and heading, flowering and filling) and the density of microorganisms. A high number of Azotobacters and Actinomycetes were noticed during the tillering stage compared to the other vegetative stages such as bolting/heading and the filling stage. The lowest rates were recorded at the emergence stage. These results are consistent with those found by (Davet, 1996), who presented a similar study on the variation of the soil microorganism’s number in the rhizosphere associated with durum wheat cultivation. The intense activity of photosynthesis recognizes the tillering stage. This activity is more intense for irrigated crops than non-irrigated crops. This explains the increase in the number of microorganisms during the tillering stage. During the other stages, when the plant exhibits a decrease in photosynthetic activity, a decrease in microorganisms is noticed. It is apparent that at the tillering stage, each microbial germ reaches its maximum. Similar results have been obtained in previous works (Oulbachir, 2010).
Water stress affects the physicochemical parameters of the soil. There is a correlation confirmed statistically by the correlation matrix between the studied set of parameters such as the correlation between the rate of humidity and total limestone, the rate of limestone and pH, the rate of organic matter and density of soil microorganisms, the rate of organic matter and vegetative stages. This is illustrated by the set of interactions responsible for maintaining the ecosystems (Baize, 2018). These correlations explain all of the variations in the growth of the living soil organisms, as there are interactions between all abiotic and biotic soil components. The rate of organic matter gradually decreases with the growth of the crop and the vegetative stages. Meager rates of organic matter have been noticed at the end of the planting cycle. These rates were low in the cultivated soil; lower than the bare soil rates. Also, they were low for the irrigated crop; lower than the non-irrigated crops. The statistical study, via the correlation matrix, shows a positive correlation between the rate of organic matter and the number of most soil microorganisms. Organic matter is the source of nutrition for plants and soil microorganisms regardless of its origin (Raven, 2009).
Conclusion
Water stress affects the rhizospheric microbial biomass, the soil parameters, and the plant production of cereal cultivation. This effect results from the disturbance of all the biotic and abiotic components of the soil, where there is a set of interactions between soil, plant, and microorganisms.
- The study of these microorganisms' behavior in association with the plant under conditions of water stress can help choose the auxiliary microorganism PGPR the most resistant to lack of water. Antagonistic at pathogenic microorganisms in the soil is capable of transforming organic matter.
- Soil parameters vary under water stress conditions. Their variation influences microbial life and plant production.
- It should be noted that there is an impact of variations in pH values on the number of fungi. This variation is due to the biological activity of all bacteria and the variation in soil parameters under water stress conditions.
- Altered plant production due to water stress can affect the microbial life of the rhizosphere; vice versa, the microbial life in the soil influences plant production.
The obtained results are used favorably to improve plant production by changing organic matter and its availability and protecting the plant against pathogenic germs that affect it such as fungi to minimize the use of phytosanitary products. These remarks can be beneficial for improving plant production in a sustainable biological way through the use of beneficial microorganisms in the phytoprotection and the evolution of organic matter. Also, they are helpful to control the conditions of water stress for the plant. Furthermore, they facilitate the choice of the most favorable conditions for plant production by monitoring the variations in the physicochemical parameters of the soil under water stress. Other in-depth studies must confirm this study over several years and on several types of durum wheat or plants in order to obtain very accurate and applicable results to ensure human nutrition and to preserve his environment.
Acknowledgements
We should like to extend our sincere thanks and deep appreciation for all those who contributed to the realization of this manuscript, particularly Mrs. Tiphaine Chevallier, Mrs. Bouabdelli Fatiha, and all engineers of the "Technical Institute of Great Cultures".
References
Adda, A ., Soualemi, S., labdelli, A., Sahnoun, M., Merah, O. (2013). Effect of water deficit on the structure of the piliferous zone of the seminal roots of durum wheat. Ecologie-Environnement, 9 : 1112-5888.
Baize, D.( 2018). Guide to soil science analyzes. Quae, Parie, 22-119.
Baize, D.( 2000). Guide to soil analyzes. INRA, Paris , 31- 58. Belkhodja, M., Bidai, Y. (2004). Response of Atriplex halimus L. seed germination under salt stress. Drought review, 4(15): 331-335.
Benouadah, S., Oulbachir, K., Benaichata, L., Miara, M.D., Snorek, J. (2020).Evolution of the microbial population of cultivated soil with organic matter input under semi- arid conditions (Tiaret, Algeria). Ukrainian Journal of Ecologie 10(3), 28-35.
Bresson, J.( 2013). Plant-micoororganism interaction: Involvement of the rhisobacterium Phyllobacterium brassicacerearum in Arabidopsis thaliana responses. Universite Montpellier II. Montpellier, 1-3.
Calvet, R.( 2003). Soil: properties and functions Volume 2 Physical and chemical phenomena Agronomic and environmental applications. INRA, Paris , 481.
Carnavalet, C. (2015). Soil biology and sustainable agriculture and agroecology. Agricultural France, Paris,10-211.
Clément, M.(1998). Physical analysis of soils. Technical & Documentation, Paris, 5-29.
Davet, P. (1996). soil microbial life and plant production. INRA, Paris , 13-49.
Davet, P., Francis, R.( 1997). Detection and isolation of fungi from the soil. INRA, Paris, 35.
FAO. 2011. http://www.fao.org. http://www.fao.org. [Online] 2011. [Citation: October 18, 2019.] http://www.fao.org/3/a-i1235f.pdf.
Gallais, A.( 2015). Understand the improvement of plants, issues, method, objectives and selection criteria Paris. Quae, Paris, 147-148.
Gallais, A., Bannerot, H.( 1992). Improvement of cultivated plant species, objectives and selection criteria. INRA, Paris, 27.
Gobat, J., Aragno, M., Matthey, W.( 2010). The living soil: pedology-soil biology bases. Revised and augmented, Suisse, 52-64. Lepoivre, P. (2003). Phytopathology.. De Boek Université, Bruxelles, 23-27.
Lopez, C.G., Banowetz, G.M., Peterson, C.J., Kronstad, W.E. (2003). Dehydrin expression and drought tolerance in seven wheat cultivars. Crop Sci, 43: 577–582.
Oulbachir, K. ( 2010). Microbial ecology of soils under different particle size compartments and different bioclimatic floors. doctoral thesis in agronomy, University of Oran. Algeria, 51-55.
Prescott, D ., Willey, J., Sherwood, L., Woolverton, C.( 2018). Prescott's Microbiology. [trad.] Joseleau, J.P., Perraud,R ., Coyett, J. DeBoeck superieur, Paris , 637-642.
Raven, P.H., Berg, L.R., Hassenzal D.M .( 2009). Environment. De Boeck, Bruxelle , 56- 357.
Author Info
H.Marouane1, K. Oulbachir1 and K. Acem2*2Laboratory of Plant Physiology Applied to Soilless Crops, Faculty of Nature Sciences and Life, University of Tiaret, BP 78, 14000, Tiaret, Algeria
Citation: Marouane, H., Oulbachir, K., Acem, K. (2021). Effect of water stress on the density of rhizospheric fungi, Actinomycetes, and Azotobacters associated with cereal cultivation. Ukrainian Journal of Ecology, 11 (3), 14-21.
Received: 04-Apr-2021 Accepted: 04-May-2021 Published: 29-May-2021, DOI: 10.15421/2021_136
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
