Research - (2022) Volume 12, Issue 6
Effect of hydration on renal activity in a deserticole rodent, Gerbillus tarabuli, subjected to a water-rich diet
S. Seddiki* and N. LebailiAbstract
Our work aims to study the effects of hydration (7 days) on the morpho-functional aspect of the kidneys in a rodent deserticola, Gerbillus tarabuli, well adapted to water scarcity. Fifteen adult gerbils of mixed-sex are subject to a water-rich diet (15 HG) consisting of lettuce, dates, and barley grains. During seven days, no-hydrated gerbils (15 NHG) receive only barley grains and dates. At the end of the experiment, gerbils were sacrificed, and the kidneys were intended for histological and immunohistochemical study. We examined the renal distribution and expression of aquaporins (AQP1 and AQP2) in the kidneys, and the blood was used for the determination of renal biochemical parameters. Our study's results show that hydration leads to reducing the body weight of gerbils, increasing the relative weights of the kidneys, and decreasing the plasma levels of creatinine, urea, and uric acid at the end of the 7th day. Structurally, the glomeruli have an extension in the glomerular chamber during hydration. Gerbillus tarabuli adopt a strategy to conserve body water, and this strategy is essential for adaptation and survival in dry and semi-arid desert areas. These results indicate that gerbils have renal activity that is well-suited for low-water hydration; hydration is viewed as a stress factor stimulating the renal activity in order to maintain renal hydromineral balance.
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Keywords
Hydromineral balance, Gerbillus tarabuli, Renal activity, Stress, Hydration, Aquaporins.
Introduction
The desert area is characterized by climatic conditions unfavorable to the development of most animals (high temperatures, low and irregular rainfall, etc.) (Haggag and El-Hussein, 1974). Thanks to ecological, anatomical, and physiological adaptation strategies, some desert rodents have acquired a remarkable ability to resist heat and lack of water for long periods (Haggag and El-Hussein, 1974, Degen, 1997; Walsberg, 2000; Bozinovic and Gallardo, 2006).
The previous work has focused on anatomical adaptations. Powell (1987) has showed that desert rodents have a great capacity to absorb water and electrolytes thanks to their small intestine provided with villi and developed brush border. Among these rodents, gerbils do not take free water, and they are limited to the water contained in their nutrition which is produced by metabolic oxidation (Schmidt-Nielsen, 1958; Schmidt-Nielsen, 1964; Ghobrial, 1975; Yagil, 1985). These gerbils have adapted some of their physiological functions and mainly those that contribute to reducing the depletion of water, particularly in the kidneys and the digestive tract. They produce urine concentrated in urea and electrolytes and low-water feces (Schmidt-Nielsen, 1964; Cortes et al., 1988; Palgi and Haim, 2003).
The comparative study of the morphology of the kidney in some desert species has shown certain structural particularities which could reflect an adaptation to their arid environment (Rouffignac et al., 1969; Barrett et al., 1978; Rouffignac et al., 1981, Meng-Meng and De-Hua, 2016). The kidney of the gerbil, Gerbillus tarabuli exhibits a long, narrow renal papilla and low glomerular density, which contributes to the production of concentrated, low-volume urine (Saadi and Lebaili, 2012; Nemiri and Ouali-Hassenaoui, 2020).
Aquaporins (AQPs), a family of membrane proteins which function as water channels, play a crucial role in water reabsorption along the nephron in mammals (Nielsen et al., 2002; King et al., 2004). Among the 8 AQPs distributed in kidney, the knockout of AQP1, AQP2 generates a severe urine-concentrating defect in rats and mice (Verkman, 2009; Kortenoeven and Fenton, 2014).
The physiological mechanism of how gerbils cope with water stress remains unclear. It has been established that the up-regulation of AQP2 expression is a water-sparing mechanism at the molecular level which occurs under conditions of negative water balance.
This leads to an increased urine concentrating capacity at the organismal level (Nielsen et al., 1993; Yang et al., 1999; Nielsen et al., 2002; Bozinovic and Gallardo, 2006). There has been little investigation into the function and regulation of renal AQPs in desert rodents (Bozinovic and Gallardo, 2006), and, consequently, studies on a wider variety of species would further our understanding of this urine-concentrating mechanism (Pannabecker, 2013).
Given the crucial role of the kidneys in the regulation and maintenance of hydromineral balance, we proposed in the present work to seek the involvement of renal activity as a morpho-physiological adaptation strategy in the gerbil, Gerbillus tarabuli. This research is based on the study of the repercussions that hydration, through a diet rich in water, can have on the morphological and functional aspect of the kidneys.
Materials and Methods
Animals and experimental design
All animal procedures were licensed and manipulations were performed according to the recommendations of the Algerian ethical committee and under the supervision of authorized investigators.
Thirty adult male and female gerbils (G. tarabuli), weighing between 32 g and 53 g, from Algerian arid zones station (Beni Abbes region). These gerbils were divided in to two groups each group consists of gerbils (n=15). After gerbils were acclimatized for 48h prior to the start of the experiment, the animals were housed individually in plastic cages (30 cm x 15 cm x 20 cm ) with sawdust as bedding under a constant photoperiod of 14 h and temperature of 24° ± 1° and relative humidity (20-25%).
The firstly group, no -hydrated gerbils (15NHG) were given barley grain (100 g containing: 10.4% water; 9.6% protein; 50.3% sugar; 50.3% cellulose; total mineral content: 2.7%) and dry dates (100 g containing: 15% water; 2.5% protein; 69% sugar; total mineral content: 1.5 to 1.8 g; 7 g fiber) during seven days. The secend group, hydrated gerbils (15HG) were given barley grain; dry dates and fresh lettuce leaves (100 g containing: 95% water; 1.7 g protein; 2% sugar; total mineral content: 2 to 2.6 g: 1% fiber) ad libitum, during seven days, Under the rich-water diet, the gerbil orients his dietary choice to wards the lettuce.
Body and relatives kidney weights RKW
The animals were weighed in the morning of the first day and in the end of experiment using a balance (KERN EW420-3NM, Germany). The Kidney were weighed individually with precision balance (KERN ABT 220-5DM, Germany).
Measurement of morphological and physiological parameters
At the end of the experiment, the sacrifices take place in the morning between 9 h and 11 h, the kidneys are removed and intended for a histological and immunohistochemical study, the arteriovenous blood is collected, either on heparin or on EDTA, then centrifuged for 15 min. at 3000 rpm within two minutes. The plasma collected is intended for the determination of creatinine, urea and uric acid.
Immunolocalization of AQPs
The kidneys fixed in Bouin’s solution, were paraffin-embedded (7 mm thick). Localization of AQP1 and AQP2 was done by immunohistochemistry following the procedures of (Gallardo et al., 2005). The monoclonal primary antibodies were Mouse anti-AQP1 (diluted 1: 200, 1/A5F6, Thermo Fisher Scientific (Invitrogen Life Technologies), USA), The polyclonal primary antibodies were Rabbit anti-AQP2 (diluted 1:1OO, 1/A5F6, Thermo Fisher Scientific (Invitrogen Life Technologies), USA).
Statistical analysis
The results are presented as mean ± SD. An analysis of the variance and the Student t-test were performed for the comparison of the no-hydrated gerbils group and the hydrated gerbils. The differences are considered significant when P<0.05. We will represent three classes of results:
* P<0.05
** P<0.01
*** P<0.001
Results and Discussion
Weight Effects
Effect on body weight: Hydrated gerbils demonstrate a regression in body weight at the end of the 7th day (44.52 ± 2.01 g) compared to no-hydrated gerbils (40.75 ± 1.12 g). This difference is statistically insignificant (P>0.5) (Fig. 1).
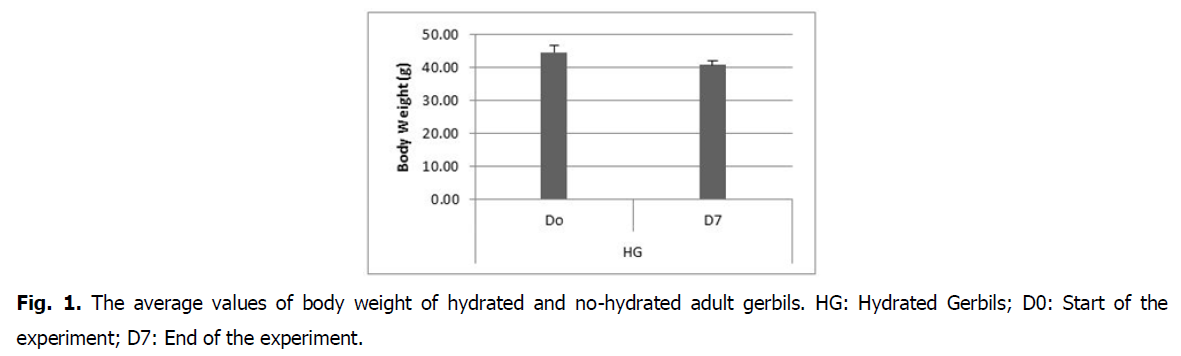
Fig 1: The average values of body weight of hydrated and no-hydrated adult gerbils. HG: Hydrated Gerbils; D0: Start of the experiment; D7: End of the experiment.
Effect on kidney weight: The absolute weight (182.3 ± 3.5 mg) and relative kidney weight (446.9 ± 5.8 mg%) in hydrated gerbils increased compared to absolute weight (172.3 ± 2.7 mg) and the relative weight (408.5 ± 9.3 mg%) in no-hydrated gerbils (Fig. 2 and Fig. 3).
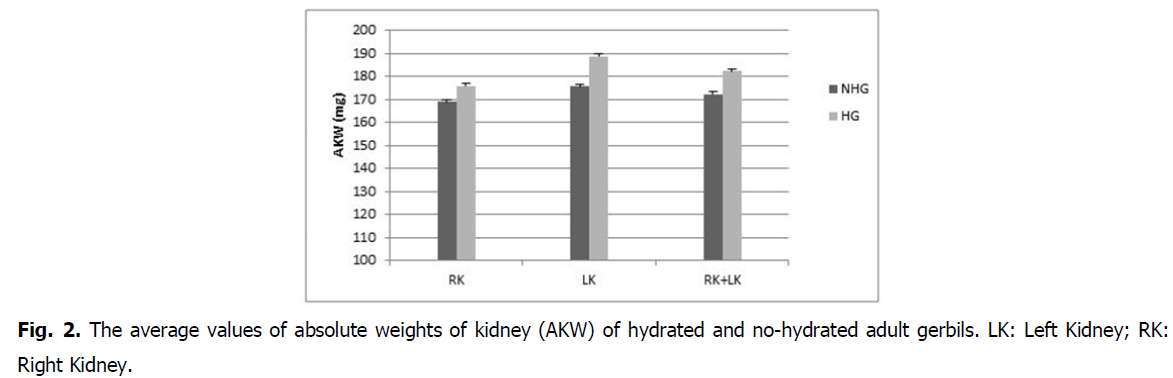
Fig 2: The average values of absolute weights of kidney (AKW) of hydrated and no-hydrated adult gerbils. LK: Left Kidney; RK: Right Kidney.
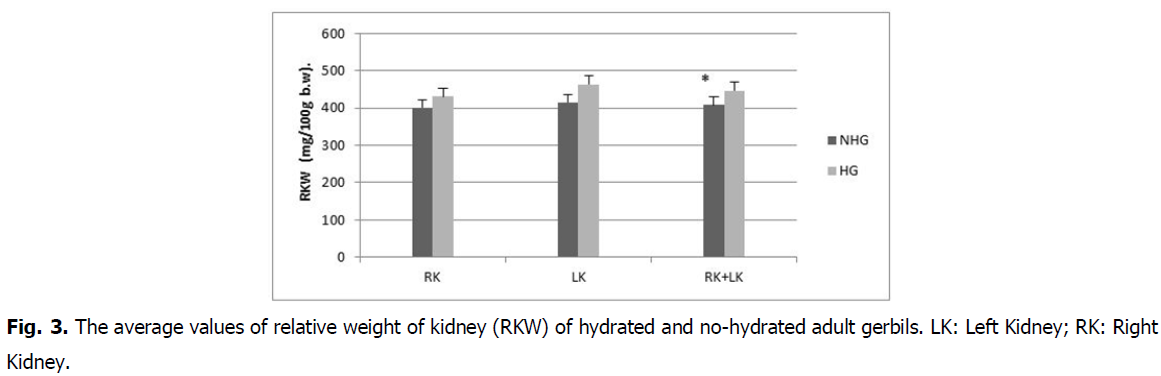
Fig 3: The average values of relative weight of kidney (RKW) of hydrated and no-hydrated adult gerbils. LK: Left Kidney; RK: Right Kidney.
The difference is statistically insignificant (P>0.5) in absolute weight and statistically highly significant in relative weight (P<0.01).
Effects on diuresis and renal plasma parameters
Effects on diuresis: In hydrated gerbils, a statistically highly significant increase (P<0.01) in diuresis (9272.7 ± 71.7 µl/24 h) compared to no-hydrated gerbils (411.1 ± 8.4 µl/24 h) is observed (Fig. 4).
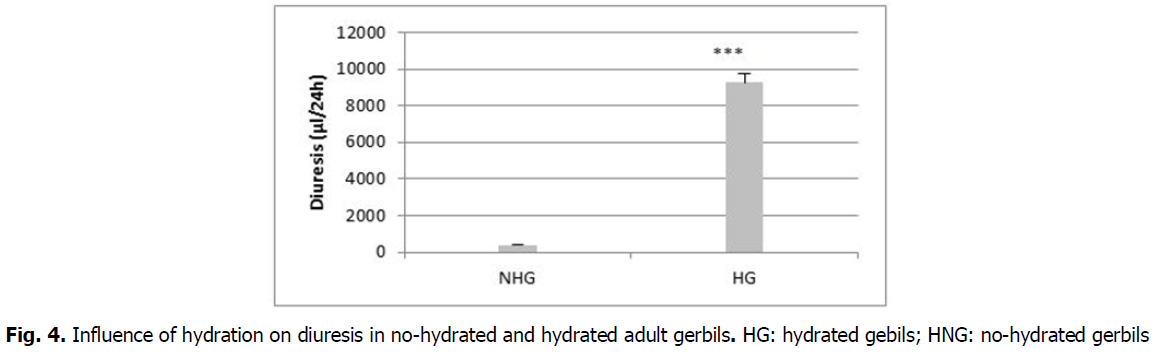
Fig 4: Influence of hydration on diuresis in no-hydrated and hydrated adult gerbils. HG: hydrated gebils; HNG: no-hydrated gerbils
Effects on plasma kidney parameters
Hydration causes a decrease in plasma creatinine concentration (3.16 ± 0.18 mg/l) compared to no-hydrated gerbils (4.19 ± 0.16 mg/l). This decrease is statistically highly significant (P<0.01) (Table 1).
| Comparative groups | Effectives | Creatinine Pl. (mg/l) | Urea Pl. (g/l) | Uric acid Pl. (mg/l) |
|---|---|---|---|---|
| No-hyrated Gerbils | 15 | 4.19 ± 0.16 | 0.281 ± 0.01 | 2.53 ± 0.13 |
| Hydrated Gerbils | 15 | 3.16 ± 0.18 | 0.244 ± 0.01 | 0.33 ± 0.08 |
Table 1. Plasma Kidney Parameters (Creatinine, Urea and Uric acid) in Gerbillus tarabuli under water-rich diet and dehydration.
A statistically significant (0.05<P<0.02) decrease in plasma urea concentration (0.244 ± 0.01 g/l) was observed in hydrated gerbils compared to no-hydrated gerbils (0.281 ± 0.01 g/l) (Table 1).
Plasma uric acid concentration decreased in hydrated gerbils (0.33 ± 0.08 mg/l) compared to no-hydrated gerbils (2.53 ± 0.13 mg/l). This decrease is statistically highly significant (P<0.01) (Table 1).
Kidney Histology and Immunohistochemistry
Kidney histology
• Glomeruli are ovoid and surrounded by a reduced Bowman's space (glomerular chamber) in (HNG) no hydrated Gerbils (Fig. 5 and Fig. 6).
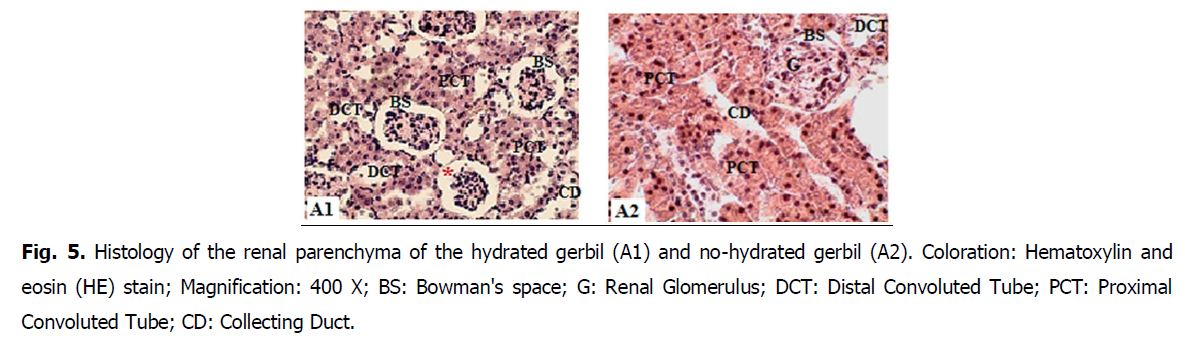
Fig 5: Histology of the renal parenchyma of the hydrated gerbil (A1) and no-hydrated gerbil (A2). Coloration: Hematoxylin and eosin (HE) stain; Magnification: 400 X; BS: Bowman's space; G: Renal Glomerulus; DCT: Distal Convoluted Tube; PCT: Proximal Convoluted Tube; CD: Collecting Duct.
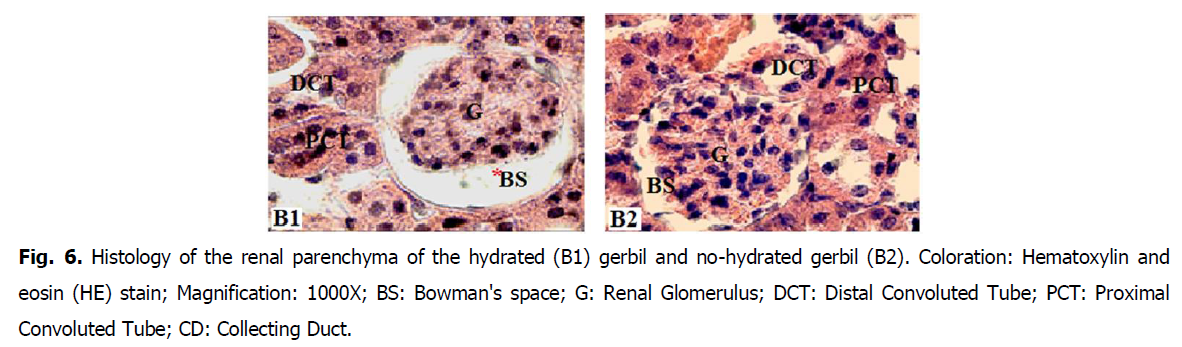
Fig 6: Histology of the renal parenchyma of the hydrated (B1) gerbil and no-hydrated gerbil (B2). Coloration: Hematoxylin and eosin (HE) stain; Magnification: 1000X; BS: Bowman's space; G: Renal Glomerulus; DCT: Distal Convoluted Tube; PCT: Proximal Convoluted Tube; CD: Collecting Duct.
• The analysis of the sections at the level of kidney parenchyma of the hydrated gerbils, the glomeruli demonstrate a remarkable dilation of the glomerular chambers, Bowman's space (Fig. 5 and Fig. 6)
• Most of the tubes surrounding the glomeruli are proximal convoluted tubule (PCT) with a reduced lumen and are more numerous than the distal tubule (DCT) (Fig. 5 and Fig. 6).
• The medulla is taken over mainly by loops of Henlé: the presence of a large number of slender branches and wide ascending branches (Fig. 7).
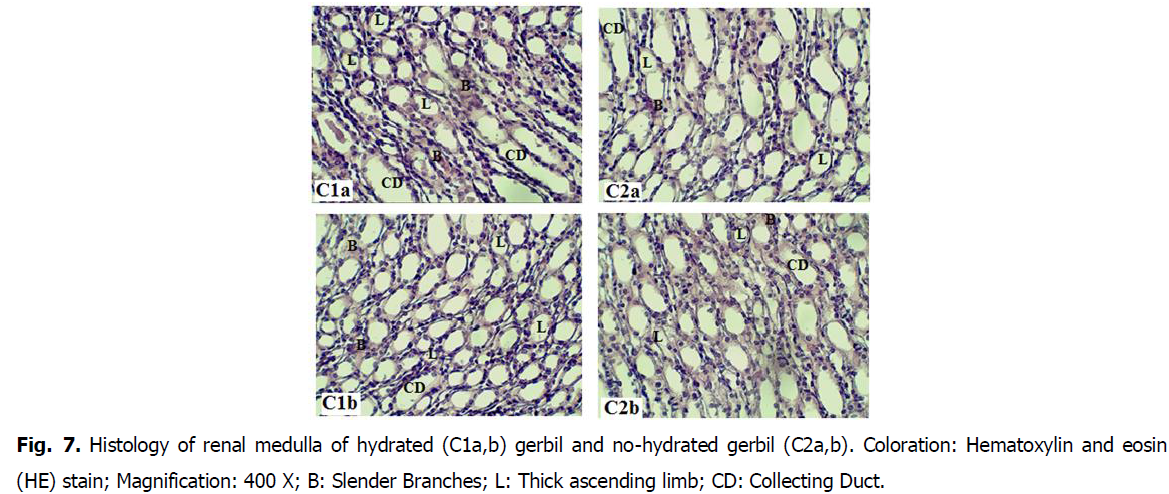
Fig 7: Histology of renal medulla of hydrated (C1a,b) gerbil and no-hydrated gerbil (C2a,b). Coloration: Hematoxylin and eosin (HE) stain; Magnification: 400 X; B: Slender Branches; L: Thick ascending limb; CD: Collecting Duct.
Immunolocalization and abundance of AQP1, AQP2
• Immunostain of AQP1and AQP2 was much lighter in the cortex than the medulla.
• Where we observed the presence of AQP1 in the proximal tubules in the cortex and the descending thin limbs in the medulla.
• AQP2 was more abundant in hydrated gerbils (HG) than no-hydrated gerbils (HNG), and it was correlated by a twice increase in AQP2 abundance in hydrated gerbils (Fig. 8-11).
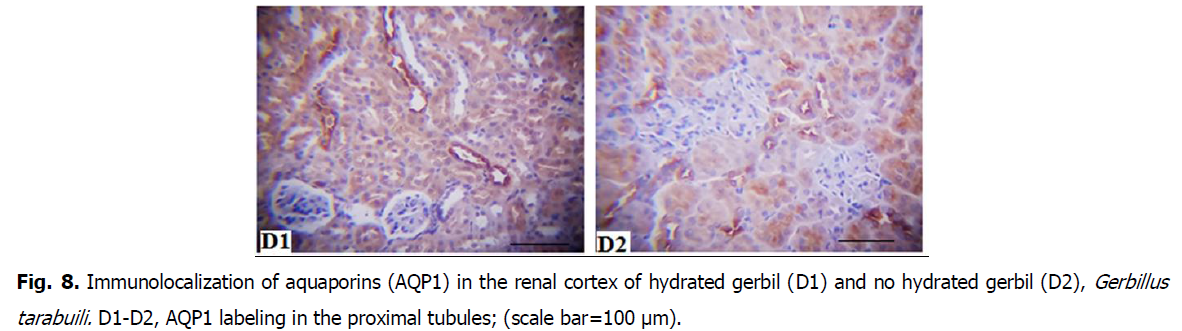
Fig 8: Immunolocalization of aquaporins (AQP1) in the renal cortex of hydrated gerbil (D1) and no hydrated gerbil (D2), Gerbillus tarabuili. D1-D2, AQP1 labeling in the proximal tubules; (scale bar=100 μm).
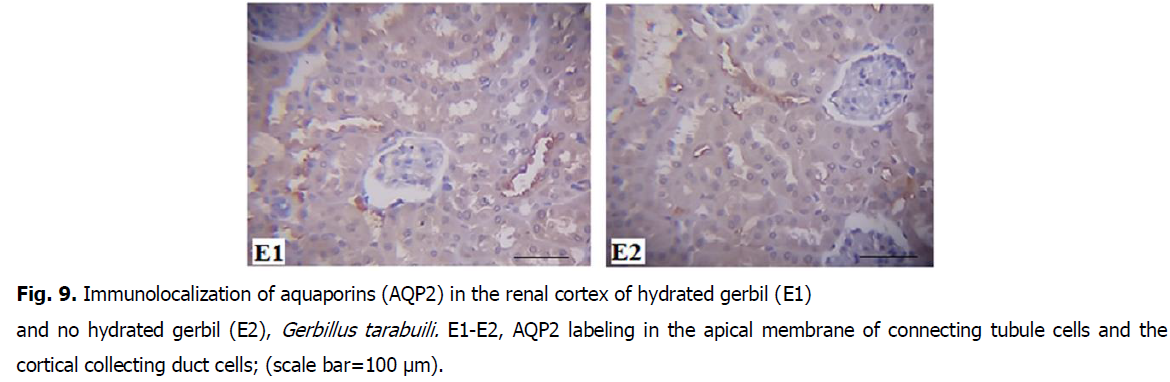
Fig 9: Immunolocalization of aquaporins (AQP2) in the renal cortex of hydrated gerbil (E1) and no hydrated gerbil (E2), Gerbillus tarabuili. E1-E2, AQP2 labeling in the apical membrane of connecting tubule cells and the cortical collecting duct cells; (scale bar=100 μm).
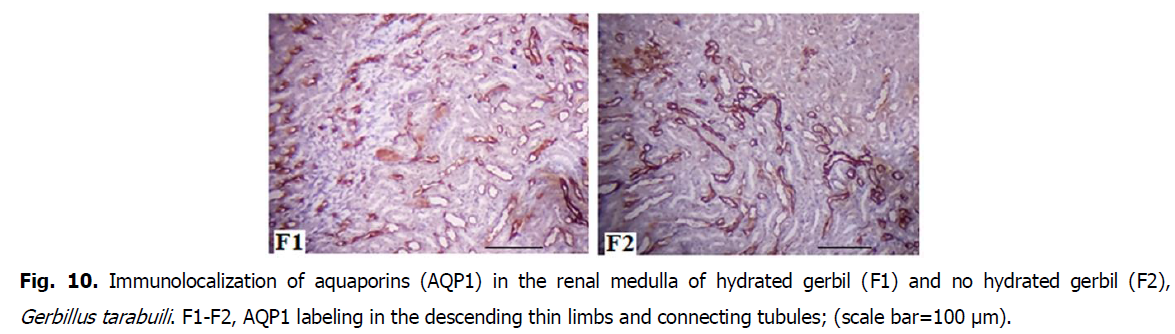
Fig 10: Immunolocalization of aquaporins (AQP1) in the renal medulla of hydrated gerbil (F1) and no hydrated gerbil (F2), Gerbillus tarabuili. F1-F2, AQP1 labeling in the descending thin limbs and connecting tubules; (scale bar=100 μm).
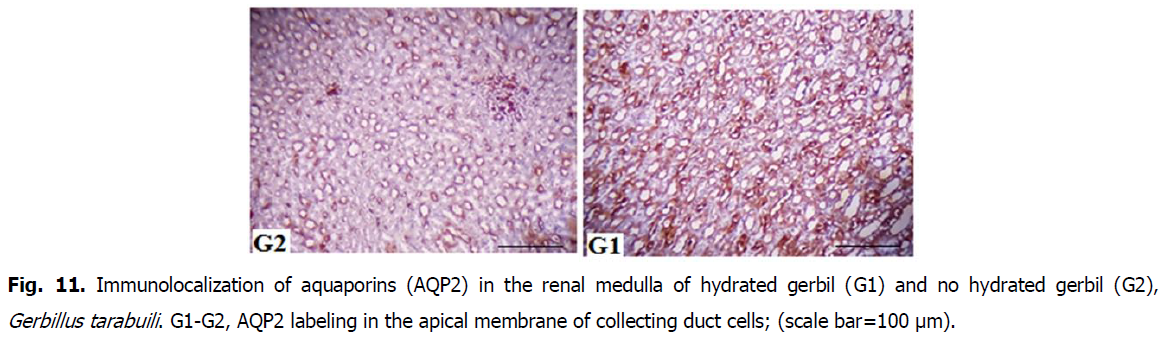
Fig 11: Immunolocalization of aquaporins (AQP2) in the renal medulla of hydrated gerbil (G1) and no hydrated gerbil (G2), Gerbillus tarabuili. G1-G2, AQP2 labeling in the apical membrane of collecting duct cells; (scale bar=100 μm).
Discussion
Hydrated gerbils showed a statistically non-significant retrogression in body weight on the seventh day of hydration (Saadi and Lebaili, 2002; Meng-Meng and De-Hua, 2016). This is probably due to the change in feeding behavior by the animal. Gerbils consume lettuce, avoiding barley grains and dates. Contradictory results have been reported by (Saadi, 2001) and (Donath et al., 1999) and Similar results are recorded in the gerbil, Mongolian gerbils, by (Meng-Meng and De-Hua, 2016).
Hydration keeps this dimorphism and leads to a statistically non-significant increase in the absolute weight of both kidneys and a statistically highly significant increase in relative weight compared to those of the no hydrated gerbils. This increase may probably reflect the presence of a trophic impact on kidney morphology during hydration. Similar results are recorded in the gerbil, Gerbillus tarabuli, by (Zatra, 2008).
Hydrated gerbils showed a considerably higher diuresis rate than no hydrated gerbils. This result reflects the activation of water-saving mechanisms and concentrated urine production in the no hydrated gerbil and the ability to keep low-water metabolism in the hydrated gerbil(Saadi and Lebaili, 2002; Meng-Meng and De-Hua, 2016). (Ben Chaouacha-Chekir, 1989) showed that in desert rodents such as the Meriones shawi shawi, the decrease in water metabolism and urine concentration allows them not to modify their volume of body water. The elimination of urine from the kidney depends on the hormones controlling the transport mechanisms at the level of the nephrons, hormones among which vasopressin and aldosterone are the significant contributors (Yagil et al., 1979; Blair-West et al., 1980; De Mccormick et al., 2006).
The activity of gerbils subjected to a water-rich diet showed a decrease in the plasma creatinine, urea, and uric acid concentration compared to that of the no hydrated gerbils. This change is attributed to hemodilution probably accompanied by an increase in renal glomerular filtration and a decrease in tubular reabsorption. These results are similar to those found by (Abdelatif et al., 2010) in which the rate of plasma urea of Namibian goats tends to decrease after 24 hours of rehydration. This decrease is explained by the non-recycling of urea during hydration and the decrease in tubular reabsorption of urea followed by the decrease in circulating vasopressin (Yagil et al., 1979; Blair-West et al., 1980). The latter promotes the reabsorption of urea in the renal tubes.
Creatinine is the best endogenous marker of glomerular filtration and good renal function for a long time (Tsinalis and Binet, 2006). The high concentration of creatinine in no hydrated gerbils could be related to the maintenance of moderate renal function and consequently a reduction in the rate of urine. Creatinine concentration considerably decreased in hydrated gerbils probably reflects the increase of glomerular filtration and urinary excretion to take off the water excess introduced during hydration and adjust the water balance.
The statistically highly significant decrease in uric acid rate in hydrated gerbils can be explained by a large excretion of nitrogen with the urine in response to increased water supply in the body.
The results of renal activity corroborate those of the histological study. According to the analysis of the sections at the level of the kidney parenchyma of the hydrated gerbils, the glomeruli showed a remarkable dilation of the chamber, Bowman's space (Nemiri and Ouali-Hassenaoui, 2020). These results probably confirm the increase in glomerular filtration during hydration to release the water excess provided by the lettuce and to ensure the return of the water balance to a normal state.
AQP1 is the constitutive water-specific channel, and AQP2 is present on the apical membrane in collecting duct cells, where the final fine-tuning of water and urea reabsorption is achieved (Nielsen et al., 2002; Kortenoeven and Fenton, 2014). Similar to other rodents, renal AQP1 abundance of gerbils (Gerbillus tarabuli) was unaltered, suggesting that it plays a constitutive role in water absorption (Nielsen et al., 2002).
The up-regulation of renal AQP2 in no-hydrated gerbils is consistent with the leaf-eared mouse following 5 days of water deprivation (Gallardo et al., 2005), free-living degus during summer (Bozinovic et al., 2003), and rats and mice after short periods of water deprivation (Nielsen et al., 1993; Yang et al., 1999). It seems that desert rodents show a generality of AQP1 and AQP2 responses to water deprivations similar to rats and mice, and AQP2 plays an important role of hydromineral balance. AQP1 is present in the proximal tubules in the cortex and the descending thin limbs in the medulla. Neither immunoreactivity nor AQP1 abundance differed between groups (Meng-Meng and De-Hua, 2016).
Conclusion and Perspectives
In its biotope, the gerbil maintains its hydromineral balance by reducing the rate of urinary excretion during water deprivation. This maintenance is ensured by reduced renal activity.
Hydration can be considered as a stress factor engendering a situation of hydromineral imbalance. In response to this situation, renal activity is stimulated: increased filtration and decreased plasma rates of renal parameters see creatinine, urea, and uric acid.
Gerbillus tarabuli showed high flexibility of renal AQPs expression and distribution, to conserve body water, and this flexibility is essential for adaptation and survival in dry and semi-arid desert areas.
The research on the implication of the endocrine and metabolic systems in the body's response to hydration in Gerbillus tarabuli could have another way of research.
Acknowledgments
We are grateful to all the members of Eco-biology Animals (LEBA) for their helpful assistance and discussion. Thanks to Prof. Slimani, pathologist and head of the pathology department at CHU Béni Messous. We thank the anonymous reviewers for their helpful and constructive comments on this manuscript. This work was supported by the Higher Normal School of Kouba Bachir El Ibrahimi, Minister for Higher Education and Scientific Research of Algeria.
References
El-Latif, H.A., Ismail, E., Salem, M.H., Hasan, G. (1997). Effect of dehydration on some biochemical constituents of blood in Barki, Suffolk and their crossbred sheep. Indian Journal of Animal Sciences, 67:786-791.
Barrett, J.M., Kriz, W., Kaissling, B., de Rouffignac, C. (1978). The ultrastructure of the nephrons of the desert rodent (Psammomys obesus) kidney II. Thin limbs of Henle of long‐looped nephrons. American Journal of Anatomy, 151:499-514.
Google Scholar, Crossref, Indexed at
Ben Chaouacha-Chekir, R. (1989). ‘Fonction Thyroidienne et Métabolisme Hydrique chez quelques Gerbillidés du Sud Tunisien. These doct. d’état, Muséum National d’Histoire Naturelle et Université Pierre et Marie Curie, Paris, 6.
Blair-West, J.R., Bobik, A., Brook, A.H., Esler, M.D., Gibson, A., Morris, M., Pullan, P.T. (1980). Renin, ADH and the kidney: a congeries of conundrums. Progress in Biochemical Pharmacology, 17:20-28.
Bozinovic, F., Gallardo, P. (2006). The water economy of South American desert rodents: From integrative to molecular physiological ecology. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology, 142:163-172.
Google Scholar, Crossref, Indexed at
Cortes, A., Zuleta, C., Rosenmann, M. (1988). Comparative water economy of sympatric rodents in a Chilean semi-arid habitat. Comparative Biochemistry and Physiology-Part A: Physiology, 91:711-714.
Google Scholar, Crossref, Indexed at
McCormick, S.D., Bradshaw, D. (2006). Hormonal control of salt and water balance in vertebrates. General and Comparative Endocrinology, 147:3-8.
Google Scholar, Crossref, Indexed at
Degen, A.A. (1997). Water requirements and water balance. In Ecophysiology of Small Desert Mammals. Springer, Berlin, Heidelberg, pp:93-162.
Donath, M.Y., Gross, D.J., Cerasi, E., Kaiser, N. (1999). Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes, 48:738-744.
Gallardo, P.A., Cortés, A., Bozinovic, F. (2005). Phenotypic flexibility at the molecular and organismal level allows desert-dwelling rodents to cope with seasonal water availability. Physiological and Biochemical Zoology, 78:145-152.
Google Scholar, Crossref, Indexed at
Ghobrial, L.I., Nour, T.A. (1975). The physiological adaptations of desert rodents. In Rodents in Desert Environments, Springer, Dordrecht, pp:413-444.
El-Husseini, M., Haggag, G. (1974). Antidiuretic hormone and water conservation in desert rodents. Comparative Biochemistry and Physiology Part A: Physiology, 47:347-350.
Google Scholar, Crossref, Indexed at
King, L.S., Kozono, D., Agre, P. (2004). From structure to disease: The evolving tale of aquaporin biology. Nature Reviews Molecular Cell Biology, 5:687-698.
Kortenoeven, M.L., Fenton, R.A. (2014). Renal aquaporins and water balance disorders. Biochimica et Biophysica Acta (BBA)-General Subjects, 1840:1533-1549.
Google Scholar, Crossref, Indexed at
Xu, M.M., Wang, D.H. (2016). Water deprivation up-regulates urine osmolality and renal aquaporin 2 in Mongolian gerbils (Meriones unguiculatus). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 194:37-44.
Google Scholar, Crossref, Indexed at
Nemiri, N., Ouali‐Hassenaoui, S. (2020). Anatomical, histological and biochemical studies of desert rodent Gerbillus tarabuli (Thomas, 1902) kidney. Anatomia, Histologia, Embryologia, 49:486-493.
Google Scholar, Crossref, Indexed at
Nielsen, S., Smith, B.L., Christensen, E.I., Knepper, M.A., Agre, P. (1993). CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. The Journal of Cell Biology, 120:371-383.
Google Scholar, Crossref, Indexed at
Nielsen, S., Frøkiær, J., Marples, D., Kwon, T.H., Agre, P., Knepper, M.A. (2002). Aquaporins in the kidney: from molecules to medicine. Physiological Reviews, 82:205-244.
Google Scholar, Crossref, Indexed at
Palgi, N., Haim, A. (2003). Thermoregulatory and osmoregulatory responses to dehydration in the bushy-tailed gerbil Sekeetamys calurus. Journal of Arid Environments, 55:727-736.
Google Scholar, Crossref, Indexed at
Pannabecker, T.L. (2013). Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: role of inner medullary water and urea transport pathways in the rodent medulla. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 304:R488-R503.
Google Scholar, Crossref, Indexed at
Powell, D.W. (1987). Intestinal water and electrolyte transport. Physiology of the Gastrointestinal Tract, 2:1267-1305.
De Rouffignac, C., Bankir, L., Roinel, N., Philippe, P., Soyeux, N., Malorey, P. (1981). Renal function and concentrating ability in a desert rodent: the gundi (Ctenodactylus vali). Pflügers Archiv, 390:138-144.
Google Scholar, Crossref, Indexed at
Rouffignac, C., Morel, F., Marsh, M., Guinnebault, M. et Lechene, C. (1969). Etude par microponction de l'é1aboration de l'urine. II. Chez le Psammomys nondiuréique. Néphron, 6:553-570.
Saadi, L. (2001). Effet de la déshydration et de l'hydratation sur la cytologie de la zine glomérulée corticosurenalienne chez un rongeur saharien la grande gerbille (Gerbillus pyramidum). (Doctoral dissertation, Ecole normale supérieure de Kouba-Mohamed Bachir El Ibrahimi).
Saadi, L., Lebaili, N. (2012). Effect of a water-rich diet on adrenal zona glomerulosa in Gerbillus tarabuli. Comptes Rendus Biologies, 335:96-102.
Google Scholar, Crossref, Indexed at
Schmidt-Nielsen, K. (1964). Desert animals. Physiological problems of heat and water. Desert Animals. Physiological Problems of Heat and Water.
Schmidt-Nielsen, B. (1958). Urea excretion in mammals. Physiological Reviews, 38:139-168.
Tsinalis, D., Binet, I. (2006). Appréciation de la fonction rénale: créatininémie, urée et filtration glomérulaire. In Forum Med (Suisse), 6:414-419.
Verkman, A.S. (2009). Knock-out models reveal new aquaporin functions. Aquaporins, 359-381.
Google Scholar, Crossref, Indexed at
Walsberg, G.E. (2000). Small mammals in hot deserts: Some generalizations revisited. Bioscience, 50:109-120.
Google Scholar, Crossref, Indexed at
Yagil, R., Etzion, Z. (1979). The role of antidiuretic hormone and aldosterone in the dehydrated and rehydrated camel. Comparative Biochemistry and Physiology Part A: Physiology, 63:275-278.
Yagil, R. (1985). The desert camel. Comparative physiological adaptation. Karger.
Yang, B., Ma, T., Xu, Z., Verkman, A.S. (1999). cDNA and genomic cloning of mouse aquaporin-2: Functional analysis of an orthologous mutant causing nephrogenic diabetes insipidus. Genomics, 57:79-83.
Google Scholar, Crossref, Indexed at
Zatra, Y. (2008). Influence de la castration, en période de reproduction, sur l'activité du cortex surrénal chez la gerbille male.
Author Info
S. Seddiki* and N. LebailiCitation: Seddiki, S., Lebaili, N. (2022). Effect of hydration on renal activity in a deserticole rodent, Gerbillus tarabuli, subjected to a water-rich diet. Ukrainian Journal of Ecology. 12:47-55.
Received: 22-Jul-2022, Manuscript No. UJE-22-70035; , Pre QC No. P-70035; Editor assigned: 25-Jul-2022, Pre QC No. P-70035; Reviewed: 04-Aug-2022, QC No. Q-70035; Revised: 09-Aug-2022, Manuscript No. R-70035; Published: 15-Aug-2022, DOI: 10.15421/2022_384
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
