Research - (2022) Volume 12, Issue 6
Effect of Ulva rigida as crude and formulated extract on vineyard growth at an early stage
A. Mohammedi1*, B. Zebib2, S. Atika1, O. Merah2 and Z.E. Djazouli1Abstract
Following the ecological waves that requires chemical restrictions to assure sustainable agriculture, trash from marine lifeis used as fertilizer: Ulva rigida one of the species no documented as a bio stimulant on young stage of vine, so for that we have used it to prepare a crude extract, where the quantity of algae is reduced to 70% and 60% in order to get Formulation 1 (F1) and Formulation 2 (F2) by adding surfactants. Those bio-products are diluted according 8 ml/L (v/v) and evaluated by foliar application at 50mL per plant, when nine leaves well developed (stage 11 of BBCH scale) to evaluate growth and photosynthetic parameters compared to the control (water). Our results shows that all treated vines in the early stages with seaweed extract (CRUDE EXTRACT, F1 and F2) increased growth parameters. Indeed, the dry weight was enhanced by 50% for both formulations compared to control (water), when the higher amount of leaf area showed for F2. Total pigmentations were enhanced by the F2 35.61 (mg/g FM) after the crude extract 40.37 (mg/g FM) treatment which had the double value compared to the untreated sample. The formulation composition secure the bioactive compounds of the crude extract with a double efficiency on some parameters, a potential way to get cheap foliar fertilizer and help to develop ecologically sustainable agricultural systems. The current study can open up a various investigations to examine the effect of these extracts and formulations on grapevines at different ages, their impact on growth and yield on vine, even on others crops.
Keywords
Bio-stimulant, Ulva rigida, formulation, crude extract.
Introduction
The vine (Vitisvinifera L.) has a great economic importance (Fraga, 2019). The world’s total vineyard surface area in 2007 was estimated at around 7.5 million ha (Issacs R, 2012) according to Ceev, 2020 the EU is the first producer, consumer, exporter and importer in the wine world representing 45% of world’s wine growing area, 57% of global wine consumption 63% of global win production, 70% of total win export, following the economic indicators analysis’s (TO and FNVA) French holdings are consistently above the EU average, making them the leaders in Europe (Pappalardo et al., 2013). This species is among the most treated with chemicals in order to assure an economical production. Nevertheless, vine cultivation, still needs use chemicals which did not meets the European Union recommendations. Indeed, the EU had 3.2 million hectares in 2015 Breakdown of area planted with vines that represented around 45% of the world’s total area under vines (Costa, 2019) which still fell under the generalization of intensive agriculture and the use of chemical fertilizers, have a serious impact on human health (Rengasamy et al., 2015).
The limitation of chemicals, in the recent years, has induced the necessity to develop surrogates for agriculture uses. There is growing reflections about enhancing the agricultural crops by following the ecological waves and using safe fertilizers, such as seaweed source, which are considered like trash from a marine life, with a great importance gaining over commercial synthetic fertilizer (Khan et al., 2009); owing to their biodegradability without harming the environment (Craigie, 2010).
It is therefore crucial to develop economically and agronomically viable alternatives to the restrictions of chemical inputs (Merah et al. 2021). Numerous organic substances have been suggested as a source of nutrients and stimulants for cultivated plants. Seaweed, humic substances and compost are the most promising sources of biostimulants (Sharma et al., 2014; Cataldo et al. 2022; El-Sheikha et al., 2022). Studies have highlighted that extracts of different species of seaweed enhancing crop’s growth, root development, stimulation of chloroplasts concentration, plant vigor and pathogens resistance, increasing yield and seedling for on numerous crops such as tomato (Hernández-Herrera et al., 2013), peper and aubergine (Demir et al., 2018; Khan et al, 2018),onion(Abbas et al., 2020), lettuce (Chrysargyris et al., 2018) , potato (Lola-Luz et al., 2014), date palm (Anli 2020), maize (Basavaraja et al., 2018), canola (Ferreira and Lourens, 2013), spinach (Xu and Leskovar, 2015), radish (Mahmoud et al., 2019); Vigna species (Selvam and Sivakumar, 2013; Vasantharaja et al., 2019) fruit quality in Malus species (Basak, 2008; Wang et al., 2016). Seaweed extracts classified also as a“bio-stimulant” or acts as an elicitor of several phenolic compounds (Gutierrez-Gamboa et al., 2020; Cataldo et al., 2022) .
The most new researches are about enriching it with different compounds to achieve a high efficiency, as the application of amino acids(Khan et al., 2018) and iron (Álvarez-Fernández et al., 2007). All those aspects have been attributed to its richness with various components (Gupta and Abu-Ghannam, 2011) phenolic compounds (Rajauria, 2018) nutrients, vitamins, minerals, amino acids, pigments, complex polysaccharides that are not present in terrestrial plants (Hamed et al., 2018; Khan et al., 2009; Pena-Rodriguez et al., 2011) that contribute to plant growth and fruits protection (Salim et al. 2020).
In viticulture, an overview of use of bioastumilants, including seaweed extracts has been widely presented by Cataldo et al., (2022). Seaweed extracts have been used to incraease grape yield (Arioli, T et al., 2021), to control diseases in vine (Aziz, A et al.,2003) to modify the anthocyanin (Salvi, L et al.,2019; Petoumenou, D.G.; Patris, V.E., 2021) or volatil biosynthesis (Gutiérrez-Gamboa, G et al., 2020; Gutiérrez-Gamboa, G. et al., 2020; Gutiérrez-Gamboa, G. et al., 2021). Few reports studied effect of foliar applications of macroalgae extracts on growth especially under stressed conditions (Salvi, L. et al.,2020; Abbas, M et al., 2020; Frioni,T., et al., 2021; Meggio et al., 2020). Sabir et al. (2014) used aschophyllumnodosum extract and have examined its effect on growth, quality and other biochemical traits of grapvine. All these studies used extracts of different macrolagae species on aged grapevine plants. There is no study on young vine plants in order to enhance vigor and therefore instalation. Moreover, the majority of these studies used Ascophyllum nodosum, Laminariadigitata, Durvillaeapotatorum. No studies have been reported on Ulva rigida.
Ulvaceae, widely available in Mediterranean sea (Wichard et al., 2015) received less attention. The few studies done on this species have shown that chemical composition ofUlva rigida is also rich in protein, fibre,carbohydrates, vitamins and minerals, low lipid content and a good-quality protein (Taboada et al., 2009). These extracts can be applied in a different way, foliar spray (Basavaraja et al., 2018) or as soil conditioning (Eyras et al., 2008). Numerous studies havebeen carried out on the efficiency of Ascophyllumnodosum which is the most studied extract. Among all these studies have been done on vine, there is no information about the effect of Ulva rigida on earlier phenological stages as a foliar fertilizer on growth. Moreover, these studies have used crude and enriched extract so it’s a great interest to evaluate the impact of using a formulation of growth on vine.
This study aimed to evaluate the effect of the green seaweed Ulva rigida extract and its formulations on plant growth parameters of Vitis vinifera L.at earlier stages of development, young plants.
Materials and Methods
Ulva rigida was handily Collected in spring season of 2017 (end March), in the coastal regions of Bou-Ismail location (40.1 km at west of Algiers, 36° 38’ 17.517” N 2° 42’ 12.087” E Algeria) in shallow water (less than 2 m depth). The collected algae were rinsed twice by water, to avoid the presence of salt and remove impurities, sand particles, dried in ventilated oven at 40°C for 24h, and powdered by grounder.
The fresh algae taxonomically identified in Mohammedi et al.,(2021).
Preparation of aqueous extract
Water extract was performed as described by Roy et al. (2011) with some modifications. Briefly, sixty grams of powder were homogenized with 400 mL of distilled water under a background agitation at room temperature for 72 h on a magnetic stir, the macerate were centrifuged for 15 min at 4000 rpm.The supernatant was recovered and stored in the dark at 4°C in dark colored bottles. The mineral composition of the crude extract is presented in Table 2.
Preparation of formulations
The first formulation F1: is prepared by adding 70% of seaweed crude extract to a solution containing 31% of water, 5% of glycerin as wetting agent, 2% of glyceryl polyethyleneglycolricineolate (E484), as surfactant, 2% of a solution of Glucopon 215, as sticker and solubilizer agent. The mixture is then agitated with a high-speed agitator of the ultra-turrax type (Sylverson model). The resulting formulation is stored cold (4°C) in the dark.
The second formulation F2 is as prepared as the F1 by reducing the seaweed extract to 60%.
Reagent samples of glycerin, technical grade (83% purity), Bredol 696 (glyceryl polyethyleneglycolricineolate), food grade (E484), and Glucopon 215 are provided for Agronutrition.
Preparation of dilutions and application of the bio products
The both of formulations prepared with seaweed extract Ulva rigida as well as the crude extract were diluted according 8 ml/L (v/v) evaluated by foliar application at 50 mL by plant at nine leaves well developed (stage 11 of BBCH scale). This step was repeated each decade during one month.
Plant materiel, growing conditions and experimental design
The experiment was carried out twice in pots under field conditions. For each test five replications were performed, at the experimental station of University of Blida1 (North of Algeria), in Soumaa location (36°30’36.34’’ N and 2°52’26.05” E, Algeria) during the growing season of 2017. Plants were purchased from El Fartase nursery, grafted plant welded of the vine (Var RED GLOB) were transplanted into transparent plastic pots of 20 cm height and 15 cm diameter with a capacity of 1500 ml with drainage holes allowing the evacuation of the amount of excess water. The pots were filled with soil. The soil used is predominantly silty sand with the presence of clay. The organic matter content is 4.1%. The chemical composition is described in Tables 1, 2. The experimental design used is randomized complete block and the studied factor is the seaweed treatments. Water was used as a control. Plants were conducted under optimal irrigation. The irrigation was done according to the needs of the plants by adding 2L of water each two days, which corresponds to 80% of the field capacity.
| Elements | Carbonate | Bicarbonate | Chlorure | Sulfate | Nitrate | Ca | Mg | K | Na | Conductivity mS/cm | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meq/L | 0 | 6.97 | 5.01 | 7.96 | 0.17 | 9.77 | 2.7 | 0.02 | 3.4 | 1.25 | 6.9 |
Table 1. Physico-chemical parameters of the used soil in our study.
| Nutrient | Crude extract | |
|---|---|---|
| Minerals | Fe (mg/L.) | 0.041 ± 0.0025 |
| Mn (mg/L.) | 0.001 ± 0.0001 | |
| Zn (mg/L.) | 0.004 ± 0.0002 | |
| Cu (mg/L.) | 0.001 ± 0.0001 | |
| Mg (mg/L.) | 1.155 ± 0.0693 | |
| Ca (mg/L.) | 1.072 ± 0.0643 | |
| K (mg/L.) | 1.063 ± 0.0638 | |
| Pb (mg/L.) | 0.012 ± 0.0007 | |
| Cr (mg/L.) | 0.016 ± 0.0010 | |
| Cd (mg/L.) | 0.019 ± 0.0011 | |
| Ni (mg/L.) | 0.093 ± 0.0056 | |
| Pigments | Chl a (µg/g.MF) | 13.650 ± 0.8190 |
| Chl b (µg/g. MF) | 9.613 ± 0.5768 | |
| Chl Totale (µg/g.MF) | 23.263 ± 1.3958 | |
| Caroténoïdes (µg/g. MF) | 6.252 ± 0.3751 |
Table 2 : Atomic absorption spectroscopy of the crude extract Ulva rigida.
SAA Atomic absorption spectroscopy analyses of the crude extract of U. rigida 1.5 g of the algal crude extract was wet to star digestion by adding the following acids drop by drop (7 ml of HNO3 65%,1 ml of H2O2 30%) then gently the solution was swirl to homogenize the sample with the acids and nserted into the microwave cavity which was connected with the temperature sensor. The rotor was cooled by water until the solution reaches room temperature, which was transferred to a marked flask. The solution, was analyzed by Atomic absorption spectroscopy, SAA (Agilent AA Duo 240 FS/240 Z).
Weather conditions
The place is characterized through Mediterranean to sub-continental climatic conditions (cool and rainy winters). Precipitation, particularly in iciness andspring,is characterised through great inter-annual irregularity. The coldest month is January (average temperature of 7.8°C). The warmest months are July and August (common temperature of 21°C). The season of our study period (2018-2019) was very rainy with 945.8 mm which exceeded the 42-year average precipitation by almost 718 mm. In addition, the distribution of rainfall was not even. In fact, more than 82% of the rainfall fell between September 2018 and February 2019. The decrease in precipitation coincided with a high evaporative demand due mainly to increased temperatures and radiation. All Variation of daily minimum and maximum temperature and daily precipitation per year at the study site (Soumaa region) was performed by the METEONORM software, version 7.1 (Fig. 1 and 2).
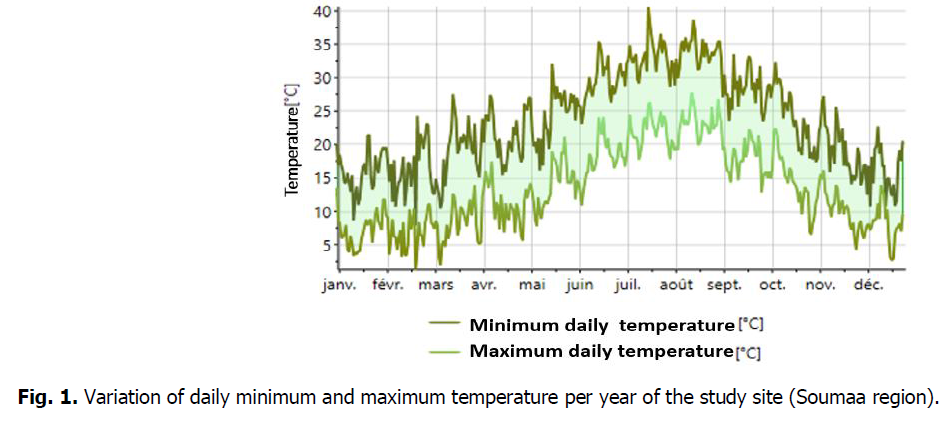
Fig 1: Variation of daily minimum and maximum temperature per year of the study site (Soumaa region).
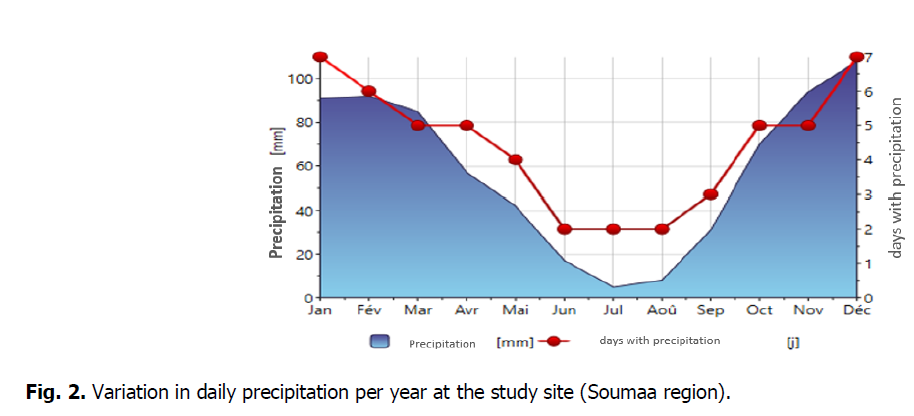
Fig 2: Variation in daily precipitation per year at the study site (Soumaa region).
Measurements
Before each treatment applications, the number of newly emergent leaves was noted. Two leaves were harvested for physiological and chemical determination.
The leaf area
It was expressed in cm2, was determined using the image analysis software Digimizer ver. 3.0. (MedCalc Software bv, Ostend, Belgium).
Relative water content
The relative water content (RWC) was released according the method of Merah (2001) on ten leaves. The fresh weight (FW) was measured at excision. The dry weight (DW) was assessed after 48 h at 80°C. RWC was the calculated following the equation:
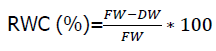
Pigments determinations
Pigments were extracted according to the method of Lichtenthaler (1987). One hundred mailgram of fresh leaves was grinded with 40ml of acetone (80%) and the extract obtained was centrifuged at 3000 rpm for 10 min. The concentration was measured by spectrophotometrically (Beckman DU 640) at 647, 664, et 470 nm.The chlorophyll a, b and carotenoïdswere calculated as:
Chla=12.21(A664)-2.79(A647)
Chlb=21.21(A647)-5.1(A664)
carotenoïds=(1000A470-1.8Chla-85.02 Chlb)/98
ChlT=Chla+Chlb
Statistical analyses
Analysis of variance was performed in order to detect the significant difference for the studied factors using ANOVA procedure of SAS (SAS Institute, 1987, Cary, NC, USA). Duncan test, at 0.5% probability level, was used to compare mean pairwise comparison.
Results
Growth traits
All treatments, in both experiments, based on seaweed extract affected significantly and positively all growth traits (Table 3). The effect of seaweed extracts was less effective in experiment 2 except for leaf area (Table 3). Formulation 2 effect was more significant that both crude extract and F1 for all traits. For example, the number of newly developed leaves and leaf area were nearly to times higher for F2 than for control. Surprisingly F1 was less efficient than crude extract of seaweed.
| Treatment | NEL | NEL | Dry Weight (g) | DW (g) | Relative water content (%) | RWC (%) | Leaf area (cm2) |
LA (cm2) |
|---|---|---|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 | |
| Water | 11.83c | 15.00b | 0.07b | 0.08b | 74.32a | 73.50a | 10.77b | 10.99d |
| Crude-extract | 19.29b | 18.42a | 0.12a | 0.11a | 80.41b | 78.64b | 18.78a | 19.55b |
| Fromulation1 | 22.33a | 18.43a | 0.09b | 0.09b | 82.01b | 84.29a | 12.03b | 14.50c |
| Fromulation2 | 21.73a | 19.88a | 0.12a | 0.12a | 81.19b | 75.49c | 20.11a | 21.71a |
Table 3. Growth parameters for both experiments (Exp1/Exp 2).
In contract RWC was higher in control compared to other treatments. All vine plants treated with seaweed extract (CRUDE EXTRACT, F1 and F2) increased the dry weight of the leaf in both experiments. Indeed, the weight dry was enhanced by 50% for both formulations compared to control (Table 3) Leaf area differences between treatments followed the same trend with higher leaf area showed for formulation 2 whatever the experiment.
The crude extract of Ulva rigida, F2 and F1 significantly increase the chlorophyll (a), the least chlorophyll contain was determined in leaf of untreated sample (11, 27 mg/g FM) when the concentration upgraded the double compared to the untreated vines with the crude extract (22, 69 mg/g FM) in the 1st experiment.In the 2nd experiment the three treatments (CRUDE EXTRACT, F1and F2) had significantly positive effect on chlorophyll (a) concentration but the highest value was for the vine treated with F2 (18.95 mg/g FM) and lowest concentration was the untreated one. 13.08 (mg/g FM) (Table 4). Chlorophyll (b), Chlorophyll (a/b) total chlorophyll and carotenoids, all increased by the formulation F2 which had the highest concentration than the untreated vin (Table 4). Total pigmentations (Table 4) were enhanced by the F2 35.61 (mg/g FM) after the crude extract 40.37(mg/g FM) treatment which had the double value compared to the control vines 20.95 (mg/g FM) in the 1st experiment but in the 2nd experiment F2 mark the highest concentration of pigments 37.84 (mg/g FM).
| Experiment | Treatment | ||||
|---|---|---|---|---|---|
| Water | Crude extrcat | F1 | F2 | ||
| Chlorophylle a mg/g FM | Exp1 | 11.27d | 22.69a | 15.51c | 17.98b |
| Exp2 | 13.08b | 18.43a | 17.94a | 18.95a | |
| Chlorophylle b mg/g FM | Exp1 | 7.44c | 13.83a | 10.77b | 13.38a |
| Exp2 | 8.06c | 13.12b | 12.92b | 15.78a | |
| Chlorophylle a/b mg/g FM | Exp1 | 1.38b | 1.83a | 1.46b | 1.99a |
| Exp2 | 1.51a | 1.41b | 1.56a | 1.54a | |
| Total Chlorophylle mg/g FM | Exp1 | 18.71d | 36.52a | 26.27c | 31.36b |
| Exp2 | 21.14b | 31.55a | 30.86a | 34.73a | |
| Carotenoïdes mg/g FM | Exp1 | 2.25d | 3.85b | 2.77c | 4.25a |
| Exp2 | 2.66b | 2.76b | 3.22a | 3.11a | |
| Total pigmentations mg/g FM | Exp1 | 20.95d | 40.37a | 29.05c | 35.61b |
| Exp2 | 23.80b | 34.31a | 34.08a | 37.84a | |
Table 4. Total pigmentations in both experiments (Exp1/Exp2).
Discussion
Seaweed extracts are considered as bio-stimulants and have several advantages over chemical imputes. They are biodegradable and safe, making them non-toxic and environmentally friendly (Gutiérrez-Gamboa, G et al., 2020). Nowadays, algae are used to provide nutrients to crops, promote the production of higher biomass and stimulate the natural ability of plants to withstand environmental factors. They are known to have a beneficial impact on plants due to their ability to produce several biologically active molecules that stimulate the plant immune system. As a result, seaweed extracts are highly recommended to help agriculture because of their very promising stable measures (Gutiérrez-Gamboa, G et al., 2020; Della Chiesa, et al., 2019; Parris, K., 2011) Understanding the effect of seaweed extracts on the growth of young vine Vitis vinifera L. is of primary importance. Indeed, the impact of fertilizer is important for growth and therefore for production (Ammad et al., 2018). The objective of our study was to examine the impact of crude and formulated extracts of a seaweed, Ulva rigida (not yet tested), on young grapevine plants to enable them to establish well.
The results obtained showed that all extracts (crude and formulated) induced an increase in growth traits (Table 3). Thus, the number of leaves was doubled for formulation 2 compared to the control. Several studies have reported positive effects of the seaweed extract’s application on plant growth and yield increase in many species such as cereal crops (Neset, T.S, et al., 2019; Omar et al., 2012; Kauffman, G.L et al., 2007; Ciavatta, C and Cavani, L., 2006), sunflower (Omar et al. 2012), legumes (Sivasankari et al., 2006, Battacharyya et al., 2015). The results of the present study are in agreement with those of many previous studies by Layek et al. Different algal species (Ulva lactuca, Caulerpascalpelliformis, Sargassummyriocystum, S. wightii, Ascophyllumnodosum) have been harvested to provide crude extract, commercial formulations and used as foliar spray or as soil amendment were tested. In all these studies, the treatments have shown growth superiority regardless of the form of application. These differences may be related to the presence of polysaccharides, vitamins, phytohormones in the seaweed extracts (Khan et al., 2009; Omar et al. 2012, Ashour et al., 2021). Unfortunately, these compounds were not measured in our work. However, seaweed sprays have shown strong growth promoting activities and acts as biostimulants in plant production with an increase in leaf number and leaf area (Table 3) This fact could be attributed to the presence of growth promoting macro and micronutrients in seaweed extracts (Sivasankari et al., 2006, Battacharyya et al., 2015). Indeed in our study, a high level of Mg was determined in the crude extract of U. rigida (Table 2). It is well known that Mg promotes the synthesis of chlorophylls since it is at the centre of the nucleus of this molecule (Mooney and van Staden, 1986).
Similar results with Ascophyllumnodosum and Ulva retuculata that have increased photosynthesis and stomatal conductance (Salvi et al., 2019) and the amount of chlorophyll a, chlorophyll b and carotenoids in both plants tested (Omar et al., 2014, Sarkar et al., 2018; Selvam and Sivakumar, 2013).
This is supported by the remarkable increase in photosynthetic pigments (chlorophylls and carotenoids) in this study (Table 4). Indeed, the treatments with the crude extract or the formulations almost doubled the contents of chlorophyll a and b and their sum as well as the carotenoids (Table 4). This increase has the effect of increasing the potential light capture by the leaves and consequently the photosynthetic activity (Omar et al., 2012) of the vines. These results are consistent with those already reported by Lakshmi and Sundaramoorthy (2010), Sangeetha and Thevanathan (2010) and Khan et al. (2012).
In grapevines, few studies have been carried out with foliar sprays of crude or formulated seaweed extracts (Ascophyllumnodosum). The various studies carried out in Turkey have shown an increase in the growth attributes of vines in production (at least 6 years old). Treatments with crude or formulated Ulva rigida extract showed similar results to those obtained by Sabir et al. (2014) with commercial Ascophyllumnodosum extracts and formulations on older vines. Similarly, Nagy and Pintér, (2014) obtained the same effects on grapevine growth using extracts of the microalgae Chlorella vulgaris. Other works have reported contradictory results. Indeed, vine is not affected by the treatments according to several studies (Frioni et al., 2019; Gutierrez-Gamboa et al., 2020a; Gutierrez-Gamboa et al., 2020b, c; Salvi et al., 2019). These differences could be attributed to the seaweed species used as well as to the age of vine plants (Sabir et al. 2014).
Similar finding have been rerported with the use of green seaweed that increased number of branches and leaves of tomato (Muthu-Pandian Chanthini et al., 2019) and cowpea with brown and red seaweed (RaguramanVasantharajaa, 2019). These treatments have improved the leaf growth even under drought stress with spinach (Xu and Leskovar, 2015; Khan et al., 2018).
Other reports have found that spinach and officinale sage treated with seaweed extracts under water stress, resulted in an increase in chlorophyll content (Kaoaua et al., 2013). Overall, under mild water stress in the present study, SWE application of U. rigida improved relative leaf water content (Table 3) and helped to maintain cell turgor pressure and maintain stomatal conductance (not measured in our study) which in turn resulted in a large leaf area and presumably, a high rate of photosynthesis, and hence increased growth.
The present study highlighted the efficiency of F2, that exhibited the highest value on almost all the parameters comparing to the crude extract, F1 and the control vines (Table 3, 4). This efficiency can be attributed to the composition of the formulation F2 (60% crude algae +40% bio adjuvants) the bio adjuvant carried the active ingredient (crude extract of Ulva rigida). Indeed, the bioadjuvant may play an important role to ensure the penetration of all those elements present in seaweed on leaves by foliar spray (Zebib et al., 2012). This fact can be due that allowed to the retention and adhesion of droplets which make a highly contact between water -leaves resulting in a good penetration on that enhances the biological activity. (Knowles, 2007; Mulqueen, 2003; Zebib et al. 2012).
Conclusion
Growth in vine at early-stage plants treated with the crude extracts of Ulva rigida formulation F1, F2 were higher than untreated plants. Foliar application of F2 on vine plants resulted in a higher growth on leaves development per plant which probably favored higher yield. This is the first report using Ulva rigida as biofertilizer. Morever, we have used the crude and formulated extract on young vines in order to help a good installation of plants. This point was not studied up to now.It appears clearly that foliar application of F2 had a beneficial effect on growth stage and leaf pigments contain of vine at young stage. Our results indicate the potential use of formulations on algae obtained from the waste of marine life as a cheap foliar fertilizer, in addition, formulation composition secure the bioactive compounds.
These results open up the prospect of increasing organic contributions to crop development, particularly in grapevines. The application of these formulations can help to develop ecologically sustainable agricultural systems; especially in areas where the use of chemical fertilizers is a financially restrictive variable. Further investigations will be needed to examine the effect of these extracts and formulations on grapevines of different ages and their impact on production, quality and response to biotic and abiotic stresses.
References
Abbas, M., Anwar, J., Zafar-ul-Hye, M., Iqbal Khan, R., Saleem, M., Rahi, A.A., Datta, R. (2020). Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae, 6:28.
Google Scholar, Crossref, Indexed at
Álvarez-Fernández, A., Pérez-Sanz, A., Lucena, J.J. (2001). Evaluation of effect of washing procedures on mineral analysis of orange and peach leaves sprayed with seaweed extracts enriched with iron. Communications in Soil Science and Plant Analysis, 32:157-170.
Anli, M., Kaoua, M.E., Boutasknit, A., ben-Laouane, R., Toubali, S., Baslam, M., Meddich, A. (2020). Seaweed extract application and arbuscularmycorrhizal fungal inoculation: a tool for promoting growth and development of date palm (Phoenix dactylifera L.) cv «Boufgous». South African Journal of Botany, 132:15-21.
Arioli, T., Mattner, S.W., Hepworth, G., McClintock, D., McClinock, R. (2021). Effect of seaweed extract application on wine grape yield in Australia. Journal of Applied Phycology, 33:1883-1891.
Ashour, M., Hassan, S.M., Elshobary, M.E., Ammar, G.A., Gaber, A., Alsanie, W. F., El-Shenody, R. (2021). Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants, 10:1045.
Google Scholar, Crossref, PubMed
Aziz, A., Poinssot, B., Daire, X., Adrian, M., Bézier, A., Lambert, B., Pugin, A. (2003). Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmoparaviticola. Molecular Plant-Microbe Interactions, 16:1118-1128.
Google Scholar, Crossref, PubMed
Basak, A. (2008). Effect of preharvest treatment with seaweed products, Kelpak® and Goëmar BM 86®, on fruit quality in apple. International Journal of Fruit Science, 8:1-14.
Google Scholar, Crossref, PubMed
Basavaraja, P.K., Yogendra, N.D., Zodape, S.T., Prakash, R., Ghosh, A. (2018). Effect of seaweed sap as foliar spray on growth and yield of hybrid maize. Journal of Plant Nutrition, 41:1851-1861.
Google Scholar, Crossref, Indexed at
Battacharyya, D., Babgohari, M.Z., Rathor, P., Prithiviraj, B. (2015). Seaweed extracts as biostimulants in horticulture. ScientiaHorticulturae, 196:39-48.
Cataldo, E., Salvi, L., Sbraci, S., Manzi, D., Masciandaro, G., Masini, C.M., Mattii, G.B. (2022). Zeowine: The synergy of zeolite and compost. Effects on the physiology of the vine and on the quality of the grapes. In BIO Web of Conferences, EDP Sciences, 44:02002.
Chanthini, K.M.P., Senthil-Nathan, S., Stanley-Raja, V., Thanigaivel, A., Karthi, S., Sivanesh, H., Soranam, R. (2019). Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatalysis and Agricultural Biotechnology, 20:101190.
Ciavatta, C., Cavani, L. (2006). Problematiche per l’inserimentodei biostimolant in ellalegislazionedei fertilizzanti. Fertilitas Agrorum, 1:11-15.
Costa, J.M., Marques da Silva, J., Pinheiro, C., Barón, M., Mylona, P., Centritto, M., Oliveira, M.M. (2019). Opportunities and limitations of crop phenotyping in southern European countries. Frontiers in Plant Science, p:1125.
Google Scholar, Crossref, PubMed
Craigie, J.S. (2011). Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology, 23:371-393.
Google Scholar, Crossref, PubMed
DellaChiesa, S., Genova, G., la Cecilia, D., Niedrist, G. (2019). Phytoavailable phosphorus (P2O5) and potassium (K2O) in topsoil for apple orchards and vineyards, South Tyrol, Italy. Journal of Maps, 15:555-562.
Google Scholar, Crossref, Indexed at
Demir, I., Ozden, E., Yıldırım, K.C., Sahin, O., Van Staden, J. (2018). Priming with smoke-derived karrikinolide enhances germination and transplant quality of immature and mature pepper seed lots. South African Journal of Botany, 115:264-268.
Google Scholar, Crossref, Indexed at
Eco-Friendly, C.P.S.R., Toy, P.P.S. (2014). 7. Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, Norrie J, Hernández-Carmona G. Effect of liquid seaweed extracts on growth of tomato seedlings Solanumlycopersicum L. Journal of Applied Phycology, 26:619-28.
Mimoun, E.K., Halima, C., Abdelali, B., Lamya, N. (2013). Seaweed liquid extracts effect on Salvia officinalis growth, biochemical compounds and water deficit tolerance. African Journal of Biotechnology, 12:4481-4489.
El-Sheikha, A.F., Allam, A.Y., Taha, M., Varzakas, T. (2022). How Does the Addition of Biostimulants Affect the Growth, Yield, and Quality Parameters of the Snap Bean (Phaseolus vulgaris L.)? How Is This Reflected in Its Nutritional Value?. Applied Sciences, 12:776.
Google Scholar, Crossref, Indexed at
Ferreira, M.I., Lourens, A.F. (2002). The efficacy of liquid seaweed extract on the yield of canola plants. South African Journal of Plant and Soil, 19:159-161.
Google Scholar, Crossref, Indexed at
Fraga, H. (2019). Viticulture and winemaking under climate change. Agronomy, 9:783.
Frioni, T., Tombesi, S., Quaglia, M., Calderini, O., Moretti, C., Poni, S., Palliotti, A. (2019). Metabolic and transcriptional changes associated with the use of Ascophyllumnodosum extracts as tools to improve the quality of wine grapes (Vitisvinifera cv. Sangiovese) and their tolerance to biotic stress. Journal of the Science of Food and Agriculture, 99:6350-6363.
Górka, B., Wieczorek, P.P. (2017). Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. Journal of Chromatography B, 1057:32-39.
Google Scholar, Crossref, PubMed
Gross, L.J., Kirschbaum, M.U.F., Pearcy, R.W. (1991). A dynamic model of photosynthesis in varying light taking account of stomatal conductance, C3‐cycle intermediates, photorespiration and Rubisco activation. Plant, Cell and Environment, 14:881-893.
Gupta, S., Abu-Ghannam, N. (2011). Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innovative Food Science and Emerging Technologies, 12:600-609.
Google Scholar, Crossref, Indexed at
Gutiérrez‐Gamboa, G., Garde‐Cerdán, T., Martínez‐Lapuente, L., Costa, B.S.D., Rubio‐Bretón, P., Pérez‐Álvarez, E.P. (2020a). Phenolic composition of Tempranillo Blanco (Vitisvinifera L.) grapes and wines after biostimulation via a foliar seaweed application. Journal of the Science of Food and Agriculture, 100:825-835.
Google Scholar, Crossref, PubMed
Gutiérrez-Gamboa, G., Garde-Cerdán, T., Rubio-Bretón, P., Pérez-Álvarez, E.P. (2020b). Seaweed foliar applications at two dosages to Tempranillo blanco (Vitisvinifera L.) grapevines in two seasons: Effects on grape and wine volatile composition. Food Research International, 130:108918.
Gutiérrez-Gamboa, G., Garde-Cerdán, T., Rubio-Bretón, P., Pérez-Álvarez, E.P. (2020). Study of must and wine amino acids composition after seaweed applications to Tempranillo blanco grapevines. Food Chemistry, 308:125605.
Google Scholar, Crossref, PubMed
Hamed, S.M., Abd El-Rhman, A.A., Abdel-Raouf, N., Ibraheem, I.B. (2018). Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef University Journal of Basic and Applied Sciences, 7:104-110.
Hamel, G. (1975). Phéophycées de France.
Isaacs, R., Vincent, C., Bostanian, N.J. (2012). Vineyard IPM in a changing world: adapting to new pests, tactics, and challenges. In Arthropod Management in Vineyards, Springer, Dordrecht, pp:475-484.
Kauffman, G.L., Kneivel, D.P., &Watschke, T.L. (2007). Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Science, 47:261-267.
Khan, R.I., Hafiz, I.A., Shafique, M., Ahmad, T., Ahmed, I., Qureshi, A.A. (2018). Effect of pre-harvest foliar application of amino acids and seaweed (Ascophylumnodosum) extract on growth, yield, and storage life of different bell pepper (Capsicum annuum L.) cultivars grown under hydroponic conditions. Journal of Plant Nutrition, 41:2309-2319.
Khan, W., Rayirath, U.P., Subramanian, S., Jithesh, M.N., Rayorath, P., Hodges, D.M., Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 28:386-399.
Google Scholar, Crossref, Indexed at
Knowles, A. (2008). Recent developments of safer formulations of agrochemicals. The Environmentalist, 28:35-44.
Google Scholar, Crossref, Indexed at
Lakshmi, S., Sundaramoorthy, P. (2010). Effect of chromium on germination and seedling growth of vegetable crops. Asian Journal of Science Technology, 1:28-31.
Lichtenthaler, H.K. (1987). [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In Methods in Enzymology, 148:350-382.
Lola-Luz, T., Hennequart, F., Gaffney, M. (2014). Effect on health promoting phytochemicals following seaweed application, in potato and onion crops grown under a low input agricultural system. Scientia Horticulturae, 170:224-227.
Google Scholar, Crossref, Indexed at
Mahmoud, S.H., Salama, D.M., El-Tanahy, A.M., Abd El-Samad, E.H. (2019). Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Annals of Agricultural Sciences, 64:167-175.
Google Scholar, Crossref, Indexed at
Mohammedi, A., Yakhlaf, M.H.S., Benkradidja, H., Merah, O., Djazouli, Z.E. (2021). Priming des Semences: Approches par l’Utilisation d’Extrait Aqueux d’Algue Verte Ulva Rigida (C. Agardh, 1823). Agrobiologia, 11:2366-2376.
Mulqueen, P. (2003). Recent advances in agrochemical formulation. Advances in Colloid and Interface Science, 106:83-107.
Google Scholar, Crossref, PubMed
Nagy, P.T., Pintér, T. (2014). Effects of Foliar Biofertilizer Sprays on Nutrient Uptake, Yield, and Quality Parameters of Blaufrankish (Vitis viniferaL.) Grapes. Communications in Soil Science and Plant Analysis, 46:219-227.
Omar, H.H., Abdullatif, B.M., Al-Kazan, M.M., El-Gendy, A.M. (2015). Various applications of seaweed improves growth and biochemical constituents of Zea mays L. and Helianthus annuus L. Journal of Plant Nutrition, 38:28-40.
Pappalardo, G., Scienza, A., Vindigni, G., D'Amico, M. (2013). Profitability of wine grape growing in the EU member states. Journal of Wine Research, 24:59-76.
Parris, K. (2011). Impact of agriculture on water pollution in OECD countries: recent trends and future prospects. International Journal of Water Resources Development, 27:33-52.
Google Scholar, Crossref, Indexed at
Peña-Rodríguez, A., Mawhinney, T.P., Ricque-Marie, D., Cruz-Suárez, L.E. (2011). Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chemistry, 129:491-498.
Google Scholar, Crossref, PubMed
Perrino, E.V., Ladisa, G., Calabrese, G. (2014). Flora and plant genetic resources of ancient olive groves of Apulia (southern Italy). Genetic Resources and Crop Evolution, 61:23-53.
Petoumenou, D.G., Patris, V.E. (2021). Effects of Several Preharvest Canopy Applications on Yield and Quality of Table Grapes (Vitis vinifera L.) Cv. Crimson Seedless. Plants, 10:906.
Google Scholar, Crossref, Indexed at
Prasad, K., Das, A.K., Oza, M.D., Brahmbhatt, H., Siddhanta, A.K., Meena, R., Eswaran, K., Rajyaguru, M.R., Ghosh, P.K. (2010). Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. Journal of Agricultural Food Chemistry, 58:4594-601.
Rajauria, G. (2018). Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. Journal of Pharmaceutical and Biomedical Analysis, 148:230-237.
Vasantharajaa, R., Abraham, L.S., Inbakandan, D., Thirugnanasambandam, R., Senthilvelan, T., Jabeen, S.A., Prakash, P. (2019). Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatalysis and Agricultural Biotechnology.
Google Scholar, Crossref, Indexed at
Sabir, A., Yazar, K., Sabir, F., Kara, Z., Yazici, M.A., Goksu, N. (2014). Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Scientia Horticulturae, 175:1-8.
Salim, D., De Caro, P., Merah, O., Chbani, A. (2020). Control of post-harvest citrus green mold using Ulva lactuca extracts as a source of active substances. International Journal of Bio-Resources Stress Management, 11:287-296.
Salvi, L., Brunetti, C., Cataldo, E., Niccolai, A., Centritto, M., Ferrini, F., Mattii, G.B. (2019). Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiology and Biochemistry, 139:21-32.
Salvi, L., Brunetti, C., Cataldo, E., Storchi, P., Mattii, G.B. (2020). Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. cv Pinot noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Applied Sciences, 10:4473.
Sangeetha, V., Thevanathan, R. (2010). Effect of panchagavya on nitrate assimilation by experimental plants. The Journal of American Sciences, 6:76-82.
Sarkar, G., Jatar, N., Goswami, P., Cyriac, R., Suthindhiran, K., Jayasri, M.A. (2018). Combination of different marine algal extracts as biostimulant and biofungicide. Journal of Plant Nutrition, 41:1163-1171.
Selvam, G.G., Sivakumar, K. (2013). Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental composition using SEM–energy dispersive spectroscopic analysis. Asian Pacific Journal of Reproduction, 2:119-125.
Sharma, H.S., Fleming, C., Selby, C., Rao, J.R., Martin, T. (2014). Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. Journal of Applied Phycology, 26:465-490.
Sivasankari, S., Venkatesalu, V., Anantharaj, M., Chandrasekaran, M. (2006). Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresource Technology, 97:1745-1751.
Taboada, C., Millán, R., Míguez, I. (2010). Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. Journal of the Science of Food and Agriculture, 90:445-449.
Wang, Y., Fu, F., Li, J., Wang, G., Wu, M., Zhan, J., Mao, Z. (2016). Effects of seaweed fertilizer on the growth of Malus hupehensis Rehd. seedlings, soil enzyme activities and fungal communities under replant condition. European Journal of Soil Biology, 75:1-7.
Wichard, T., Charrier, B., Mineur, F., Bothwell, J.H., Clerck, O.D., Coates, J.C. (2015). The green seaweed Ulva: a model system to study morphogenesis. Frontiers in Plant Science, 6:72.
Xu, C., Leskovar, D.I. (2015). Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Scientia Horticulturae, 183:39-47.
Yakhin, O.I., Lubyanov, A.A., Yakhin, I.A., Brown, P.H. (2017). Biostimulants in plant science: a global perspective. Frontiers in Plant Science, 7:2049.
Zebib, B. (2012). La bioformulation des bioactifs. Application agroalimentaire, Saarbrücken: Presses Académiques Francophones.
Author Info
A. Mohammedi1*, B. Zebib2, S. Atika1, O. Merah2 and Z.E. Djazouli12Université de Toulouse INP-ENSLACET, LOI (Laboratoire de Chimie Agro-Industriel), F-31030 Toulouse, France
Citation: Mohammedi, A., Zebib, B., Atika, S., Merah, O., Djazouli, Z.E. (2022). Effect of Ulva rigida as crude and formulated extract on vineyard growth at an early stage. Ukrainian Journal of Ecology. 12:15-25.
Received: 17-Jun-2022, Manuscript No. UJE-22-66902; , Pre QC No. P-66902; Editor assigned: 18-Jun-2022, Pre QC No. P-66902; Reviewed: 29-Jun-2022, QC No. Q-66902; Revised: 04-Jul-2022, Manuscript No. R-66902; Published: 11-Jul-2022, DOI: 10.15421/2022_380
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
