Research - (2022) Volume 12, Issue 6
Dominant male and female mate choice behaviour in albino mice Mus musculus Linnaeus, 1758
Y.A. Saleh1*, M.M. Agbali1, M. Salem1 and K.A. Saad2Abstract
Two main mechanisms of sexual selection are recognized, intrasexual selection typically involves male–male competition and Intersexual selection involves some aspect of mate choice, usually by females. Here it was investigated the effect of male dominance trait on female mate choice in the mating system of albino mice Mus musculus. Mating experiment was conducted using males of known dominance rank whereby females were able to choose among males that could see and smell each other, but could not directly interact. The results showed that measured male trait chase rate, lunge rate and aggression rate were correlated with dominant male. However, male courtship rate was not related with dominant male as well as female preferences did not correspond with male dominance. The results of this study suggested a potential intersexual conflict. Further studies are necessary in order to understand female mate choice benefits and the relative role of both mechanisms of sexual selection in albino mice mating system.
Keywords
Sexual selection, Male-male competition, Mate choice, Albino mice.
Introduction
Hierarchies of dominance are common in nature, occurring in many group of animal species (Drews 1993). Dominance is traditionally associated with access to essential resources such as food, shelter, and reproduction, rank within hierarchies of dominance can be a significant of an individual`s lifetime success (Sloman and Armstrong 2002). High-ranking individual benefit from unequal access to resources and therefore achieve a greater reproductive success, but can often incur costs associated with maintaining dominance (Ellis 1995, Gerloff et al., 1999, Launhardt et al., 2001, Widdig et al., 2004, Bradley et al., 2005). For establishment and preservation of dominance hierarchies, the show of aggression is important because, the result of antagonistic interaction (including both physical attacks, and threat displays is usually the key factor in assessing dominance status (Huntingford and Turner 1987).
For dominant males, female preferences may provide various direct benefits for selective female and their offspring (Ellis 1995, Qvarnström and Forsgren 1998), dominant male paper docx. Many species in which males compete for the resources required to attract females such as high quality breeding sites, dominant males compete to monopolize resources of high quality and thus enjoy a mating advantages (Andersson 1994). However, in some species dominance may be a weak indicator for a particular female of mate choice because, female may have to share resources with other females and in such circumstances, other indication, such as the quality of the territory and number of females who have already settled, may be relevant for female mating choice (Orians 1969). Therefore, if male resources are important to female reproductive success, female should base their mate choice on the nature of the resources directly given rather than on the status of male dominance (i.e., the ability to monopolize resources) and, several experimental studies have shown mate preference tend to be focused predominately on the nature of territory or nest site rather than on male characteristics of their mate choice (Jones 1981, Warner 1987). Although female attraction to vital resources may be the reason of why males compete for access to these resources, male dominant does not inherently need to be used in female choice as a significant cue (Casalini et al., 2009).
There are many direct copulation costs which, depending on the copulatory partner, females can be injured and sometimes killed during mating, usually by multiple competing males, in species where males are larger than females or where the sex ratio is heavily male, females tend to chose a dominant male who is effective in excluding rivals thus, the chance of being wounded is minimized (Le Boeuf and Mesnick, 1991). The effect of male-male rivalry has been suggested to accurately represent the state of a male disease since, infection will prevent a male from being dominant, consequently mating with dominant males, females may escape infection by parasites (Freeland, 1981).
Different factors affecting female mate choice in mammals. Some species of rodents prefer to mate with dominant males (Drickamer 1992, de Fraipont et al., 2003) in primates and carnivores female also appear to often favour dominant partners (West and Packer 2002, Sillero-Zubiri et al., 2004). In some rodents, females prefer to mate with unmated males, which will have a higher sperm count (Pierce and Dewsbury, 1991). In many mammals males with higher levels of testosterone are more aggressive and colourful, and females prefer to mate with such males (Setchell and Dixson 2001, West and Packer 2002).
The most convincing evidence for the occurrence of female mate choice in mammals comes from captive studies, mainly of rodents, where male competition and coercion can be minimised or even excluded (Clutton‐Brock and Mcauliffe 2009). Therefore, the aim of this study was to examine how dominance trait of potential mates affect female mate choice in Abino mice Mus musculus.
Materials and Methods
Study species and Husbandry
Mice Mus musculus Linnaeus, 1758 of albino strain are polyestrous mammals. In females, sexual maturity takes place gradually from the age of 3-4 weeks. All females older than 8 weeks are able to reproduce, exhibiting a typical cyclic sexual activity. Male puberty occurs slightly earlier, sometimes as early as 5 weeks, usually at 6-8 weeks. The female reproductive cycle, the estrous cycle, lasts 4-6 days (Guenet et al., 2015). Synchronization of the estrous cycle by the presence of a male has been reported (Whitten 1956). Animals were genetically distant individuals animals and were housed in individual tanks (35 × 23 × 22 cm) with wire mesh lids until required. Cages containing either females or males were kept on different racks and a partition between them prevented visual contact between males and females. Mice were fed with seed mix (consisting mainly of millet and some sunflower seeds) and water were provided from a dropper bottle. Each cage was contained an artificial shelter, wood shavings for bedding, straw for nest building, and at least one dried millet spray, suspended from the lid, as a source of food and behavioural enrichment, in terms of food handling and climbing.
Dominance test
Dominance was determined by using the binomial approach. To do this, two males were haphazardly selected from the stock terrarium and placed in a clear glass terrarium (28 x 35 x 58.5 cm) with a female in oestrous condition, The two males were remain together for 30 min to enable dominance to establish; the dominant male. One individual were scored as the ‘winner’ and the other as the ‘loser’, the winner were be the individual doing the chasing (one animal is aggressively pursuing another, and both are running, chasing frequently results in the animals being separated from one another), biting or lunging (one of the animals rushes towards the other once or twice, the other usually moves away from the other, and it is a signal of starting fight), or the approaching, sniffing or if the other is departing. For each dyadic pair of males, one were named as ‘dominant’ and the other as ‘subdominant’ and the rate of all male behaviour mentioned previously were recorded for 30 min. Moreover, the rate of male courtship (male gently touches or nearly touches, 1-2 mm away from contact female's face or body with its front paws, sniffing the female and approaching) were scored for 30 min. The experiment was replicated 10 times with 10 different females in oestrus and 20 males.
Female mate choice test
The experimental arena were be a clear glass terrarium (28 × 35 × 58.5 cm) separated into three chambers using opaque plastic dividers. Each divider were have a 2 × 3 cm opening which allow comfortable passage for the female (Fig. 1). The side chambers were randomly assigned in the first trial to the males. The whole arena were clean and disinfect following each trial.
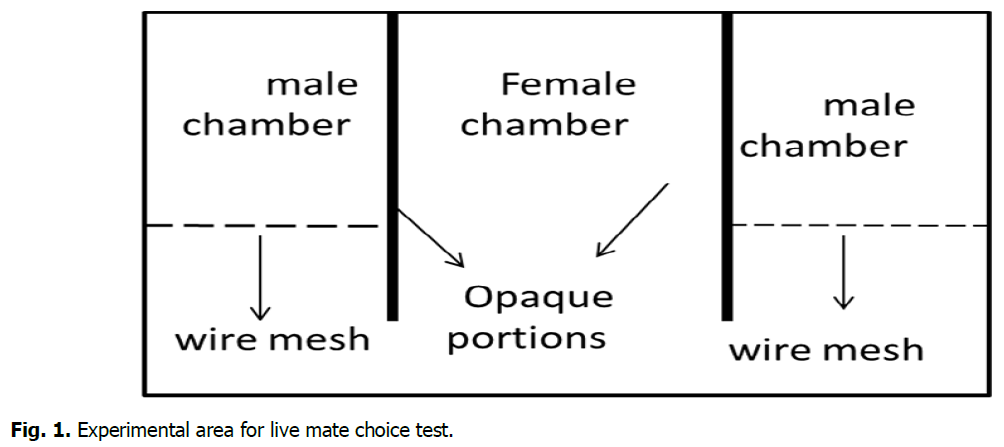
Fig 1: Experimental area for live mate choice test.
Before each trial, females were checked for oestrous condition by means of a vaginal smear (McLean et al., 2012), and were housed in the central chamber, males used in dominance experiment were assigned randomly for the side chambers, the males were leave to habituate to the chambers for an hour. Following the habituation period, the barriers between the chambers were removed allowing the female free movement throughout the arena. The animals’ behaviour were recorded for 30 min. The number and duration (to the nearest second) of the female visits to each side of the arena were recorded as measurements of mate preference. The experiment was replicated 10 times with 10 different females in oestrus and 10 males.
Statistical analysis
All data were tested for normality using a Kolmogorov–Smirnov test and for equality of variance using Bartlett’s test. Two-sample t-test and Pearson’s correlations were used to test differences and associations between behavioural data, dominance, and female mate in terms of female visiting number and duration. Two-way ANOVA was used to test all the interactions between dependent variables.
Results
The mean number of male chase rate highly varied significantly between dominant (0.2815 ± 0.018) and subdominant male (0.1070, ± 0.014) (T-Test: DF=16, T=7.52, P=0.000) (Fig. 2). In addition, there was a high significant difference between dominant (0.3497 ± 0.046) and sub dominant male(0.0617 ± 0.019) in the mean number of lung rate (T-Test: DF=11, T=5.49, P=0.000) (Fig. 3). The mean number of aggressive interactions rate shown via males differed significantly between dominant (0.1896, ± 0.022) and sub dominant male (0.5815 ± 0.018) (T-Test: DF=16, T=4.37, P=0.000) (Fig. 4). There was also a significant variation in mean number of courtship rate between dominant (0.059 ± 0.005) and sub dominant males (0.01889 ± 0.003) (T-Test: DF=13, T=6.71, P=0.000) (Fig. 5).
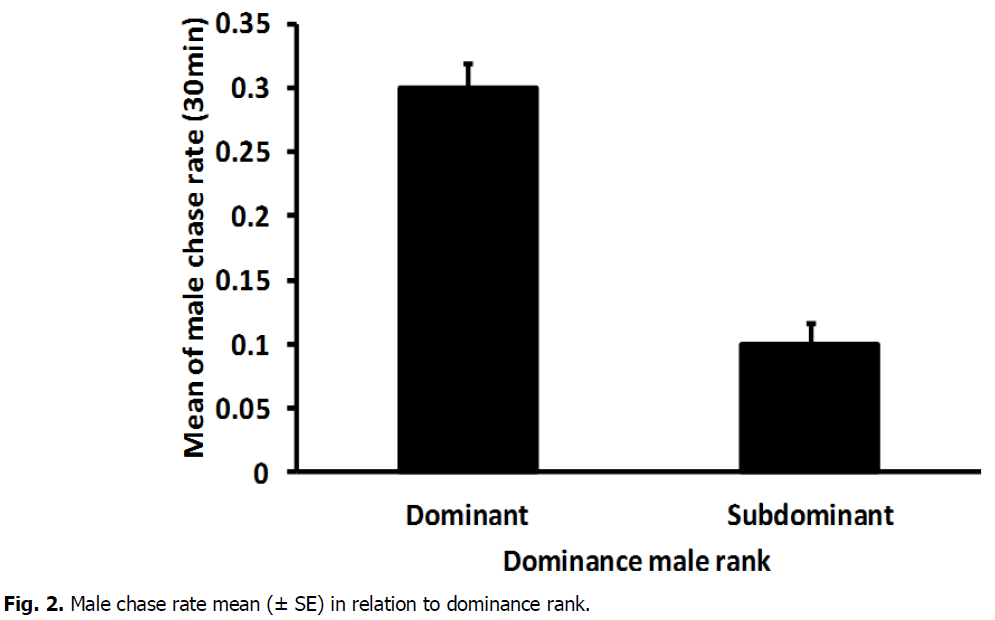
Fig 2: Male chase rate mean (± SE) in relation to dominance rank.
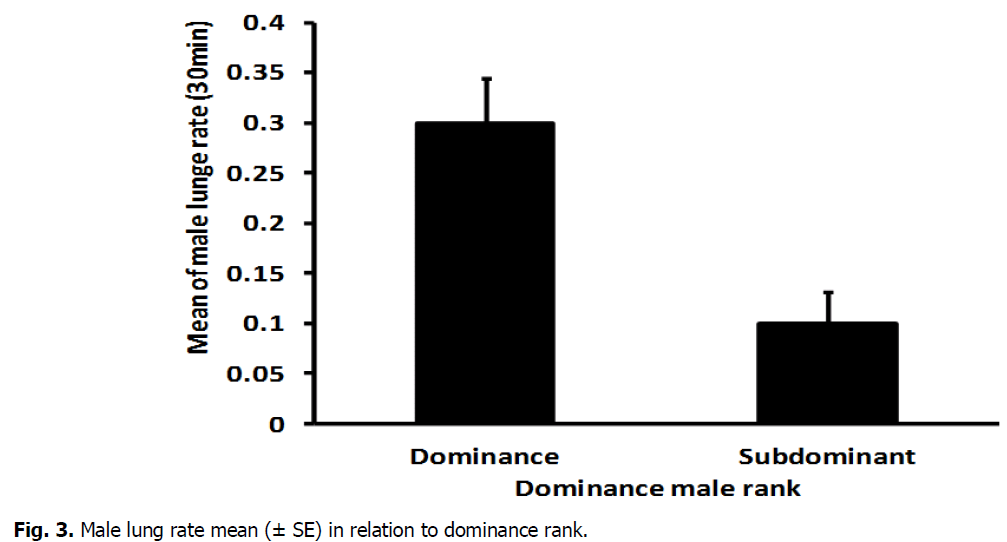
Fig 3: Male lung rate mean (± SE) in relation to dominance rank.
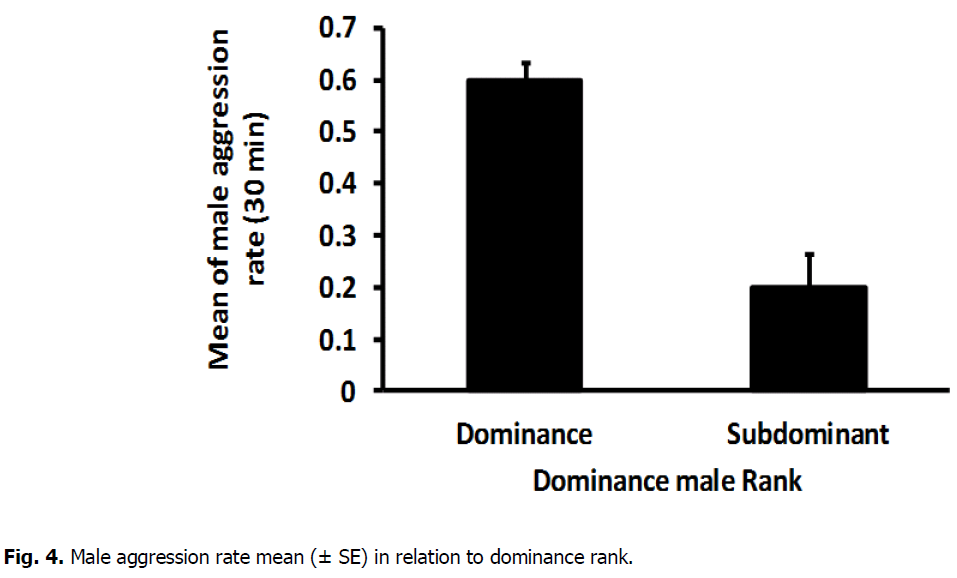
Fig 4: Male aggression rate mean (± SE) in relation to dominance rank.
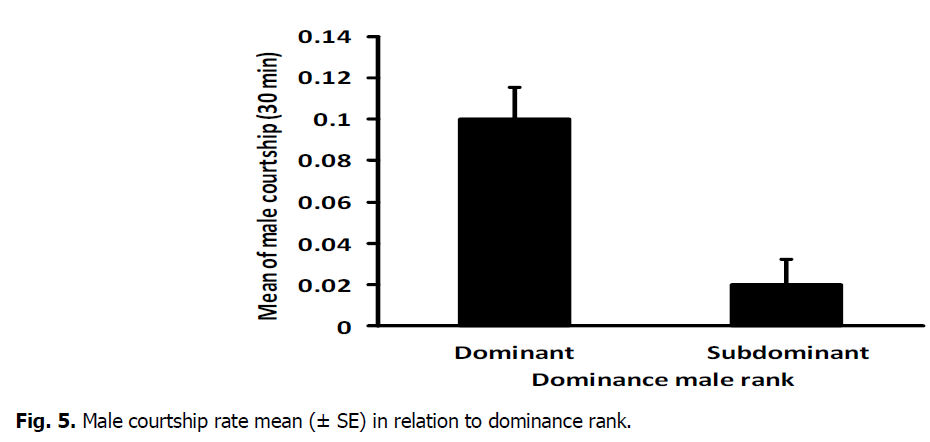
Fig 5: Male courtship rate mean (± SE) in relation to dominance rank.
The results showed that, there was a high significant difference in female visiting rate for dominant (0.045 ± 0.004) and sub dominant male (0.030 ± 0.003) (T-Test: DF=9, T=-2.68, P=0.023) (Fig. 6). As well as, the mean number of female visiting duration varied significantly between dominant (35.80 ± 1.29) and subdominant male (19.20 ± 4.06) (T-Test: DF=12, T=2.89, P=0.013) (Fig. 7).
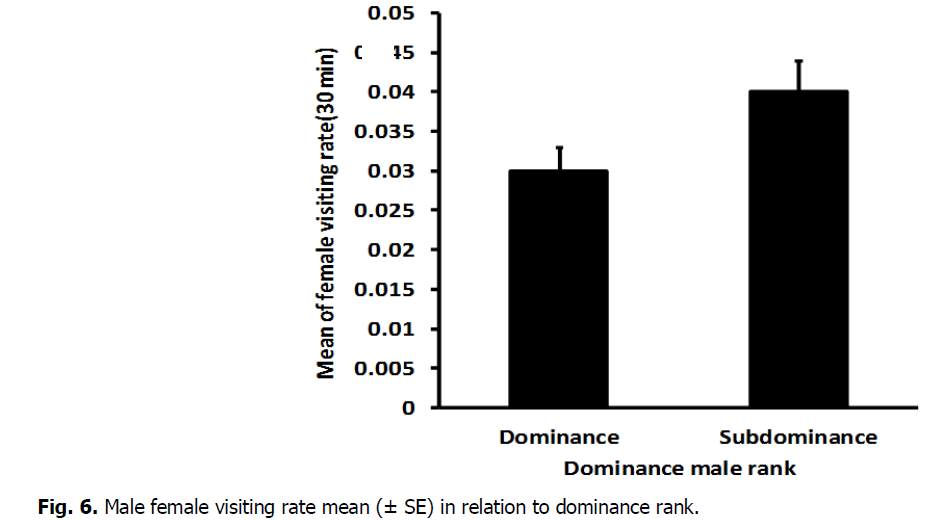
Fig 6: Male female visiting rate mean (± SE) in relation to dominance rank.
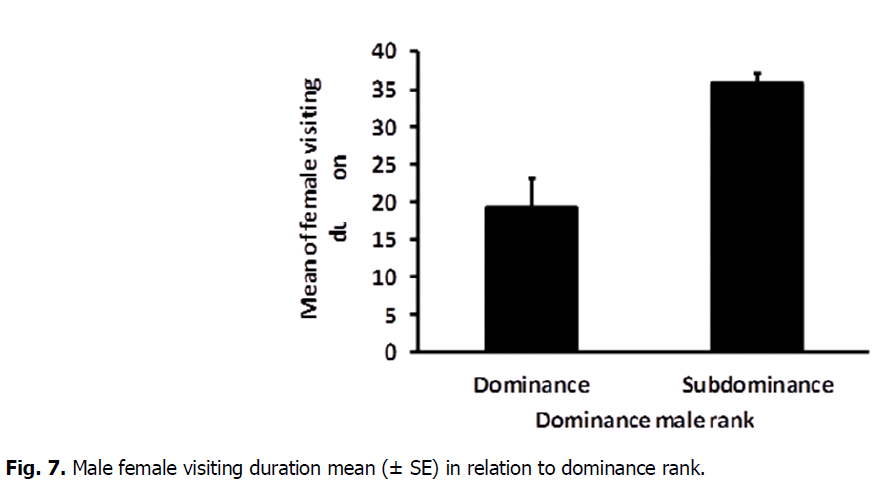
Fig 7: Male female visiting duration mean (± SE) in relation to dominance rank.
There was also no significant correlation between female visiting rate and male behavioral traits: chase rate, lung rate and aggression rate (Pearson’s correlation: chase rate r=0.128, P=0.592), (Pearson’s correlation: lunge rate r=0.152, P=0.522), and (Pearson’s correlation: aggression rate r=0.085, P=0.721) respectively. However, it was a significant correlation between female visiting rate and male courtship (Pearson’s correlation: courtship rate r=0.484, P=0.031). Furthermore, there was no significant correlation between female visiting duration and all measured male behavioural traits : chase rate, lung rate, aggression rate and male courtship (Pearson’s correlation: chase rate r=0.447, P=0.048), (Pearson’s correlation: lunge rate r=0.385, P=0.093), (Pearson’s correlation: aggression rate r=0.335, P=0.148) and (Pearson’s correlation: courtship rate r=0.423, P=0.063) respectively.
There was a significant effect of male dominance on female visiting rate and female visiting duration (Two-way ANOVA: F=5.13, P=0.037), (Two-way ANOVA: F=13.01, P=0.002) respectively (Table 1). However, there was no significant effect of male chamber (Two-way ANOVA: F=0.00, P=0.968), (Two-way ANOVA: F=0.10, P=0.761) respectively or dominance × male chamber on female visiting rate and female visiting duration (Two-way ANOVA: F=0.00, P=0.875), (Two-way ANOVA: F=1.11, P=0.308) respectively.
| Source | df | SS | MS | F | P |
|---|---|---|---|---|---|
| Female visiting rate | |||||
| Dominance (D) | 1 | 28.81 | 1241.15 | 5.13 | 0.037 |
| Male chamber (C) | 1 | 0.01 | 9.15 | 0 | 0.968 |
| D X C | 1 | 0.152 | 0.152 | 0.03 | 0.875 |
| Error | 17 | 95.39 | 5.6112 | ||
| Female visiting duration | |||||
| Dominance (D) | 1 | 1241.2 | 1241.15 | 13.01 | 0.002 |
| Male chamber (C) | 1 | 9.15 | 9.15 | 0.1 | 0.761 |
| D X C | 1 | 105 | 105 | 1.11 | 0.308 |
| Error | 17 | 1622.1 | 95.41 | ||
Table 1. The interaction effect between dominance trait and male chamber on number of female visit duration and female visiting rate.
Discussion and Conclusion
The aim of this study was to examine the interaction between male dominance and female mate choice in the albino mice Mus musculus. It was revealed that when females were capable to select males for mating without interference through male-male competition using a controlled choice experiment, females showed preferences for specific males, measured in terms of female visiting rate and duration (to the nearest second) to each side of the experimental arena. However, female mate choice did not correspond with male dominance rank, chase, lunge or aggression behaviour. The only male trait that correlated with female choice was the rate of male courtship behaviour. However, these behavioural traits were highly conducted with dominant male except courtship behaviour.
These results suggest that female mate choice may overridden by male dominance which lead to a conflict between intra and intersexual selection. It has been previously argued the conflict between female mate choice and male-male competition (Arnqvist and Rowe 2005). This study showed that male dominance did not associated with female mate choice, and similar results have been obtained for brown trout Salmo trutta (Petersson et al., 1999), and possibly in water striders Aquarius remigis (Sih et al., 2002), as less energetic costs of female mating related to small male body size. Despite harassment from dominant males, females in some species are able to mate with males that confer the highest direct or indirect fitness benefits for instance through extra-pair copulations (Mulder et al., 1994, Forstmeier et al., 2002) or avoidance of dominant males (Kangas and Lindström, 2001, Bro-Jørgensen 2003). Multiple male mating (MMM) has been observed in a variety of non-monogamous rodents such as brown rats Rattus norvegicus (Berdoy and Macdonald 2005), meadow voles Microtus pennsylvanicus (Berteaux et al., 1999), it is likely that a female mouse would also mate with more than one male in a particular oestrous period in order to maximize her fitness benefits. However, previous studies on some species of mammals have revealed an association between dominance trait and mate choice or male reproductive successes for example in fallow deer Dama dama (McElligott et al.2001), lions Panthera leo (West and Packer 2002), Ethiopian wolf Canis simensis (Sillero-Zubiri et al. 2004). Mating with a dominant male may have some advantages for the female. Uninterrupted matings, as in lekking species such as ungulates (Clutton-Brock and McAuliffe 2009) and birds (Trail 1985), and defence from male infanticide, as in many rodent species such as white footed mice Peromyscus leucopus (Wolff and Cicirello 1989), bank voles Clethrionomys glareolus (Ylönen et al. 1997). Mating with dominant males has evolutionary advantages as well. If dominance is heritable to any degree, sons of dominant males would be dominant as well, and therefore have a higher reproductive success (Drickamer 1992, Andersson 1994).
Findings of this study indicated a positive correlation between female mate choice and male courtship while also showed an adverse correlation with lunge, chase, and aggression traits of males. These results probably suggest that female mice prefer males based on less aggression rather than on active preference. Although lunge, chase, and aggression rates tended to play a role in intrasexual rivalry, there was no indication that it played a role in intersexual selection; these male traits did not predict female mate choice as females exhibited insignificant preference for dominance trait. These kinds of behavioural traits normally result in a stable dominance hierarchy which decreases violence and reduces the probability of injury of individuals (Møller and Pomiankowski 1993). Females, on the other hand, do not select dominant males or the characteristics connected with male dominance (Qvarnström and Forsgren 1998). It has been suggested that female mate preference influence the evolution of male courtship and may contribute to speciation as signals sexually selected traits also serve a role in species discrimination (Guerra and Ron 2008).
In conclusion, in albino mice Mus musculus, it was shown that intersexual selection via female mate choice and intrasexual selection via competitive male contests do not correspond. This may suggest females tend to experience a net fitness loss as a result of intrasexual selection, which possibly lead to sexual conflict. However, further researches are needed to fully understand the interaction between sexual selection mechanisms and their impact on female mate choice benefits.
References
Andersson, M.B. (1994). Sexual selection: Princeton University Press.
Arnqvist, G., Rowe, L. (2005). Sexual conflict. Princeton university press.
Berdoy, M., Macdonald, D.W. (2005). Multiple male mating in brown rats, Rattus norvegicus: male coercion or female promiscuity. WildCRU Review.
Berteaux, D., Bêty, J., Rengifo, E., Bergeron, J.M. (1999). Multiple paternity in meadow voles (Microtus pennsylvanicus): investigating the role of the female. Behavioral Ecology and Sociobiology, 45:283-291.
Bradley, B.J., Robbins, M.M., Williamson, E.A., Steklis, H.D., Steklis, N.G., Eckhardt, N., Vigilant, L. (2005). Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proceedings of the National Academy of Sciences, 102:9418-9423.
Google Scholar, Crossref, Indexed at
Bro-Jørgensen, J. (2003). The significance of hotspots to lekking topi antelopes (Damaliscus lunatus). Behavioral Ecology and Sociobiology, 53:324-331.
Google Scholar, Crossref, Indexed at
Casalini, M., Agbali, M., Reichard, M., Konečná, M., Bryjová, A., Smith, C. (2009). Male dominance, female mate choice, and intersexual conflict in the rose bitterling (Rhodeus ocellatus). Evolution: International Journal of Organic Evolution, 63:366-376.
Clutton-Brock, T., McAuliffe, B.K. (2009). Female mate choice in mammals. The Quarterly Review of Biology, 84:3-27.
Google Scholar, Crossref, Indexed at
de Fraipont, M., Berdoy, M., Rolland, C., MacDonald, D. (2003). Free female choice in house mice: leaving best for last. Behaviour, 140:1371-1388.
Google Scholar, Crossref, Indexed at
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour, 125:283-313.
Google Scholar, Crossref, Indexed at
Drickamer, L.C. (1992). Oestrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odour cues. Animal Behaviour.
Ellis, Lee. (1995). Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethology and Sociobiology, 16:257-333.
Google Scholar, Crossref, Indexed at
Forstmeier, W., Kempenaers, B., Meyer, A., Leisler, B. (2002). A novel song parameter correlates with extra-pair paternity and reflects male longevity. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269:1479-1485.
Google Scholar, Crossref, Indexed at
Freeland, W.J. (1981). Parasitism and behavioral dominance among male mice. Science, 213:461-462.
Google Scholar, Crossref, Indexed at
Gerloff, U., Hartung, B., Fruth, B., Hohmann, G., Tautz, D. (1999). Intracommunity relationships, dispersal pattern and paternity success in a wild living community of Bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266:1189-1195.
Google Scholar, Crossref, Indexed at
Guenet, J.L., Benavides, F., Panthier, J.J., Montagutelli, X. (2015). Basic concepts of reproductive biology and genetics. In Genetics of the Mouse. Springer, Berlin, Heidelberg, pp:19-49.
Google Scholar, Crossref, Indexed at
Guerra, M.A., Ron, S.R. (2008). Mate choice and courtship signal differentiation promotes speciation in an Amazonian frog. Behavioral Ecology, 19:1128-1135.
Jones, G.P. (1981). Spawning-site choice by female Pseudolabrus celidotus (Pisces: Labridae) and its influence on the mating system. Behavioral Ecology and Sociobiology, 8:129-142.
Google Scholar, Crossref, Indexed at
Kangas, N., Lindström, K. (2001). Male interactions and female mate choice in the sand goby, Pomatoschistus minutus. Animal Behaviour, 61:425-430.
Launhardt, K., Borries, C., Hardt, C., Epplen, J.T., Winkler, P. (2001). Paternity analysis of alternative male reproductive routes among the langurs (Semnopithecus entellus) of Ramnagar. Animal Behaviour, 61:53-64.
Google Scholar, Crossref, Indexed at
Le Boeuf, B.J., Mesnick, S. (1991). Sexual behavior of male northern elephant seals: I. Lethal injuries to adult females. Behaviour, 116:143-162.
McElligott, A.G., Gammell, M.P., Harty, H.C., Paini, D.R., Murphy, D.T., Walsh, J.T., Hayden, T.J. (2001). Sexual size dimorphism in fallow deer (Dama dama): do larger, heavier males gain greater mating success?. Behavioral Ecology and Sociobiology, 49:266-272.
McLean, A.C., Valenzuela, N., Fai, S., Bennett, S.A. (2012). Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of Visualized Experiments, 67:e4389.
Moller, A.P., Pomiankowski, A. (1993). Why have birds got multiple sexual ornaments?. Behavioral Ecology and Sociobiology, 32:167-176.
Mulder, R.A., Dunn, P.O., Cockburn, A., Cohen, K.A.L., Howell, M.J. (1994). Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proceedings of the Royal Society of London. Series B: Biological Sciences, 255:223-229.
Orians, G.H. (1969). On the evolution of mating systems in birds and mammals. The American Naturalist, 103:589-603.
Petersson, E., Järvi, T., Olsén, H., Mayer, I., Hedenskog, M. (1999). Male–male competition and female choice in brown trout. Animal Behaviour, 57:777-783.
Google Scholar, Crossref, Indexed at
Pierce, J.D., Dewsbury, D.A. (1991). Female preferences for unmated versus mated males in two species of voles (Microtus ochrogaster and Microtus montanus). Journal of Comparative Psychology, 105:165.
Qvarnström, A., Forsgren, E. (1998). Should females prefer dominant males?. Trends in Ecology and Evolution, 13:498-501.
Google Scholar, Crossref, Indexed at
Setchell, J.M., Dixson, A.F. (2001). Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Hormones and Behavior, 39:177-184.
Google Scholar, Crossref, Indexed at
Sih, A., Lauer, M., Krupa, J.J. (2002). Path analysis and the relative importance of male–female conflict, female choice and male-male competition in water striders. Animal Behaviour, 63:1079-1089.
Sillero-Zubiri, C., Marino, J., Gottelli, D., Macdonald, D.W. (2004). Afroalpine ecology, solitary foraging and intense sociality amongst Ethiopian wolves. The Biology and Conservation of Canids, pp:311-323.
Google Scholar, Crossref, Indexed at
Sloman, K.A., Armstrong, J.D. (2002). Physiological effects of dominance hierarchies: laboratory artefacts or natural phenomena?. Journal of Fish Biology, 61:1-23.
Trail, P.W. (1985). Courtship disruption modifies mate choice in a lek-breeding bird. Science, 227:778-780.
Google Scholar, Crossref, Indexed at
Warner, S.B. (1987). The private city: Philadelphia in three periods of its growth. University of Pennsylvania Press.
West, P.M., Packer, C. (2002). Sexual selection, temperature, and the lion's mane. Science, 297:1339-1343.
Google Scholar, Crossref, Indexed at
Whitten, W.K. (1956). Modification of the oestrous cycle of the mouse by external stimuli associated with the male. Journal of Endocrinology, 13:399-404.
Widdig, A., Bercovitch, F.B., Jürgen Streich, W., Sauermann, U., Nürnberg, P., Krawczak, M. (2004). A longitudinal analysis of reproductive skew in male rhesus macaques. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271:819-826.
Google Scholar, Crossref, Indexed at
Wolff, J.O., Cicirello, D.M. (1989). Field evidence for sexual selection and resource competition infanticide in white-footed mice. Animal Behaviour, 38:637-642.
Ylönen, H., Koskela, E., Mappes, T. (1997). Infanticide in the bank vole (Clethrionomys glareolus): occurrence and the effect of familiarity on female infanticide. In Annales Zoologici Fennici. Finnish Zoological and Botanical Publishing Board, pp:259-266.
Author Info
Y.A. Saleh1*, M.M. Agbali1, M. Salem1 and K.A. Saad22Department of Zoology, Faculty of Science, Derna University, Al Quba, Libya
Citation: Saleh, Y.A., Agbali, M.M., Salem, M., Saad, K.A. (2022). Dominant male and female mate choice behaviour in albino mice Mus musculus Linnaeus, 1758. Ukrainian Journal of Ecology. 12:6-14.
Received: 16-Jun-2022, Manuscript No. UJE-22-66806; , Pre QC No. P-66806; Editor assigned: 18-Jun-2022, Pre QC No. P-66806; Reviewed: 29-Jun-2022, QC No. Q-66806; Revised: 04-Jul-2022, Manuscript No. R-66806; Published: 11-Jul-2022, DOI: 10.15421/2022_379
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
