Commentary - (2021) Volume 0, Issue 0
Diet of Oedipoda miniata mauritanica and Oedipoda coerulescens sulfurescens(Orthoptera: Acrididae) on the coast of the Tlemcen region (Algeria)
D. Meriem*, M. Lotfi and B. NadhiraAbstract
The orthoptera represent a crucial link in food chains as primary consumers. They have long been recognized as crop pests that cause extensive damage, and thus, arouse scientific interest around their diet. In this study, our objective was to know the diet and trophic preferences of two locust species, Oedipoda miniata mauritanica (Lucas, 1849) and Oedipoda caerulescens sulfurescens (Saussure, 1884) under natural conditions. The sampling was carried out in 2016 in Ghazaouet, a coastal region of the Tlemcen subject to a subhumid climate. We examine feces contents by comparing the epidermal fragments of plants ingested by these two locusts with those of a reference collection prepared from plant species existing in the sampled biotope. This method is practical for field research, as it is simple, fast, and objective. The food spectrum of the two Oedipodinae reveals their clear preference for three plant species over all the plants present in the study area, and each species chooses its food according to its availability and nutritional characteristics. Furthermore, Oedipoda miniata mauritanica shows a great affinity for the plant species Lavendula dentata, with a consumption rate >59%, on the other hand, Oedipoda caerulescens sulfurescens prefers the plant species Lavendula multifida, with a consumption rate >50%. We can therefore consider these two locuts as euryphagous species consuming mainly Lamiaceae.
Keywords
Insecta, Oedipodinae, Feeding habit, Lamiaceae, North West Algeria, Ghazaouet.
Introduction
Food is one of the key factors for the habitat requirements of Orthoptera (Hochkirch et al., 2000). It plays a role in various biological parameters of Orthoptera populations such as fecundity, longevity, rate of development, and fertility (Dajoz, 1982). Furthermore, the overall energy value is also an element in the assessment of the quality of an aliment (Louveaux, Mainguet, and Gillon, 1983). That is how insects, in general, and locusts, in particular, select foods i.e., according to their nutritional needs. This selection occurs thanks to an extremely efficient sensory capacity that starts from the orientation of the insect towards the plant, to its bite, and its grip until its ingestion (Bennet, 1970; Louveaux, 1976).
Furthermore, a locust chooses a plant depending on the presence of certain substances that stimulate or inhibit food intake. This choice is not only linked to its own nutritional characteristics (Legal, 1989), i.e., it is not due to its nutritional value or to its abundance in the field but rather occurs according to each locust species tolerances and requirements. Once the food is detected, bites should occur and ingestion should be maintained. Food preferences may seem insignificant, yet many species starved rather than eating unusual food plants (Isely, 1944; 1946).
A better knowledge of the locust diet helps us determine whether a locust attacks the most abundant plants or the most favorite. Several works have been conducted around the world to answer this question.
By this work, we aim to: (1) define the food preference of O. miniata mauritanica and O. caerulescens sulfurescens under natural conditions in the region of Ghazouet (Tlemcen, Algeria); (2) compare the diet of these two locusts that belong to the same subfamily and genus, and see if diet changes from one Oedipodinae to another; (3) know whether or not they are able to make a food choice regarding the plant cover diversity, (4) and finally, find out if a locust adapts its feeding behavior according to the availability of plants in its biotope.
Materials and Methods
Study area
Our study area is located in the Ghazaouet region, which is located in the north-west of Algeria 80 km north of Tlemcen city. It is influenced by a Mediterranean climate characterized by a rainy winter and dry summer. This region is widely known for its richness in fauna and flora biodiversity.
We studied the food spectrum of the two Caelifera in a site located at 92 m altitude, with an exposure of 35° 06’15.93 "N and 1°50'11.67" W, on a slope of 15% and a recovery rate of 70%.
Orthoptera sampling methods
To be able to follow the Oedipodinae diet, we carried out the sampling for one year; from January to December 2016 and the frequency of field trips was two hours once a month during hot and sunny hours.
We sampled an area of around 500 m2 where the floral conditions are as homogeneous as possible. The individuals were captured with a sweeping net, placed separately in plastic bags, and fasted for 8/24 hours (sufficient time for them to empty their digestive tract) (Launois-Luong, 1976), which allows us to collect their feces.
Study method
To determine the diet of our studied species, we analysed the composition of the feces by comparing the fragments of the plants contained in the feces of the captured individuals with those of a reference epidermal library prepared from the plant species collected at the various study sites. This method has been widely used by many researchers (Ben Halima, 1983; Damerdji, Mekkioui, et Doumadji-Mitiche, 2000;El Ghadraoui, 2002; Picaud et al., 2003; Allal-Benfekih, 2006; Zaim, 2013).
Biological material
We captured 120 individuals containing 60 adult females per species. We chose females over males because they have more voluminous and numerous feces than males.
Preparation of the reference epidermal library
To establish a reference epidermal library, we sampled and used the aerial parts of each plant (stems, leaves, and flowers) present at the study site. The epidermis was prepared according to the following protocol: The epidermis was delicately detached from the underlying tissues with fine forceps, then tramped in 13% bleached water for five minutes, to be thinned in order to better see the cell walls. After being rinsed in distilled water, they were immersed in order to dehydrate them, then mounted between slide and coverslip in Faure liquid or Canada balsam, and finally the slide was quickely heated on a heating plate to avoid air bubble formation and removed to cool. We photographed the resulting slides; that constitute our epidermal libraries; using a microscope connected to a digital camera (Prat, 1932; Mohamed-Sahnoun, 1995; Kara, 1997).
Faeces analysis
The techniques for treating feces were inspired by the Launois-Luong method (Launois-Luong, 1976), which consists of softening feces for 24 hours in water with an added wetting agent (polysorbate 80) to dissociate the fragments without damaging them. Therefore, the epidermis undergoes discoloration without apparent destruction of the epidermis. The remaining steps are identical to those used for the protocol of the reference epidermal library for plants.
Ecological Indices
To study the Orthoptera diet, we used two ecological indices.
The frequency of plant species in the stool
The principle consists of noting the presence or absence of the plant in the feces; according to Butet (1985), it is expressed as follows: F(i)=ni/N × 100
F(i): Relative frequency of the epidermis contained in feces, expressed as a percentage.
ni: The number of times the plant fragments (i) are present.
N: Total number of individuals examined.
Attraction Index
This method estimates the relationship between the actual consumption of a given plant species and its recovery rate in the field. To calculate the attraction index, we used the following formulas proposed by Doumandji (1992).
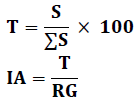
S: Average total area of a given plant species calculated for all individuals.
T: Rate of consumption of a given plant species.
IA: Index of attraction.
RG: Global coverage for a given plant species.
Results
Floristic composition
The results clearly show a very diverse floral procession (Table 1), we identified 14 plant species distributed over nine families, three of which represent the Lamiaceae family, two representatives each of Asteraceae, Liliaceae, and Poaceae. The remaining species each belong to the remaining families.
| Families | The Species |
|---|---|
| Anacardiacea | Pistacia lentiscus |
| Astéracea | Inula viscosa |
| Scolymus sp. | |
| Borraginacea | Echium vulgare |
| Cupressacea | Cupressus sempervirens |
| var. horizontalis | |
| Fabacea | Trifolium angustatifolium |
| Lavandula dentata | |
| Lamiacea | Lavandula multifida |
| Marrubium vulgare | |
| Liliacea | Asparagus officinalis |
| Asparagus stipularis | |
| Palmacea | Chamaerops humilis |
| Poacea | Avena sterilis |
| Hordeum murinum |
Table 1. Floristic inventory of the study site.
Global plant cover
The total plant cover rate of the medium is the sum of the plant cover rates of all plants present over 500 m2. This rate helps to highlight the rate of land use by dominant species and characterize the nature of the plant cover based on a scale proposed by Duranton et al. (1982).
The results of the plant cover rates evaluated at the study site (Table 2) show that L. dentata is the most representative species with a recovery rate of 32.25%, followed by P. lentiscus (12.23%) and I. viscosa (10.35%). The remaining species exhibit a relatively low cover rate.
| Station | RG% |
|---|---|
| Lavandula dentata | 32.25 |
| Pistacia lentiscus | 12.23 |
| Inula viscosa | 10.35 |
| Lavandula multifida | 7.72 |
| Marrubium vulgare | 6.01 |
| Asparagus officinalis | 3.36 |
| Hordeum murinum | 3.06 |
| Scolymus sp. | 1.72 |
| Echium vulgare | 1.63 |
| Trifolium angustatifolium | 1.52 |
| Chamaerops humilis | 1.42 |
| Asparagus stipularis | 1.21 |
| Avena sterilis | 0.83 |
| Cupressus sempervirens var. horizontalis | 0.12 |
Table 2. Overall coverage of the study area.
Locust diet
To study these two species diets, we performed a comparative analysis between the floristic composition of the plant cover of the study site and the floristic composition of the feces of the individuals that were captured in the same biotope. We calculate the ecological indices that include the frequency of plant species frequency in the faeces and the attraction index.
Oedipoda miniata mauritanica
By combining the global plant cover of the species consumed by O. miniata mauritanica, with their attraction indices and their consumption rates, we observe that the fringed lavender (L. dentata) is the most consumed species, but P. lentiscus is the most attractive species to this locust (IA=2.15), even if the global plant cover of this latter remains significantly lower than that of lavender.
At the study site, the diet of O. miniata mauritanica is composed of three plant species, L. dentata, I. viscosa and P.lentiscus. These species present an global plant cover rate of, respectfully, 32.25%, 12.23% and 10.35%.
The consumption rate remains closely related to the relative frequency of plant fragments found in the feces of O. miniata mauritanica (Fig. 1-6); we record the highest consumption rate and relative frequency value for L. dentata (T%=59.38%; F=60.52%), followed by P. lentiscus has (T%=26.38%; F%=24.87%), and finally I. viscosa which posses the lowest values(T%= 14.74%, F%15.18%).
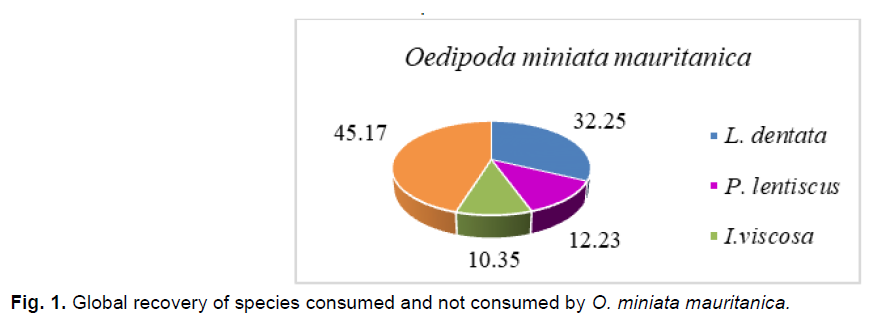
Fig 1. Global recovery of species consumed and not consumed by O. miniata mauritanica.
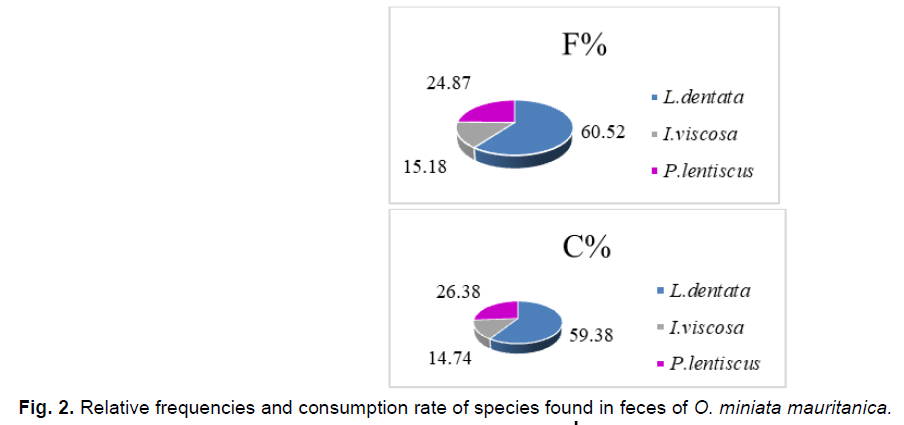
Fig 2. Relative frequencies and consumption rate of species found in feces of O. miniata mauritanica.
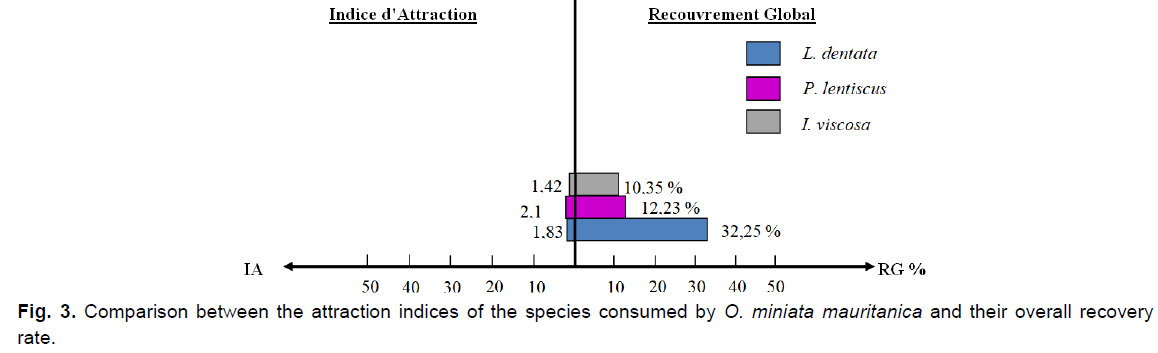
Fig 3. Comparison between the attraction indices of the species consumed by O. miniata mauritanica and their overall recovery rate.
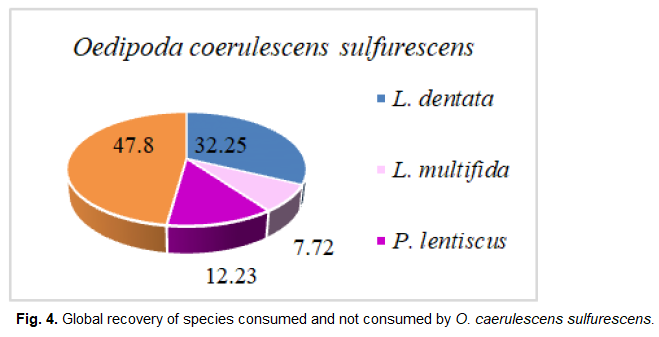
Fig 4. Global recovery of species consumed and not consumed by O. caerulescens sulfurescens.
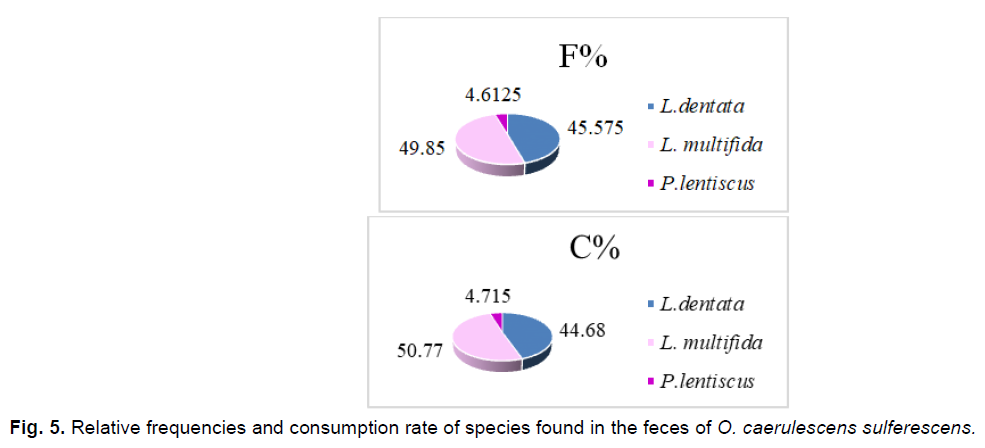
Fig 5. Relative frequencies and consumption rate of species found in the feces of O. caerulescens sulferescens.
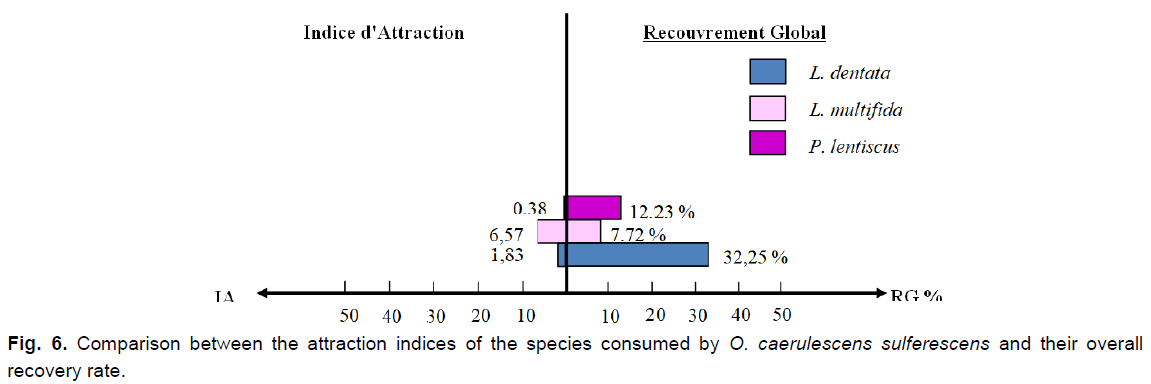
Fig 6. Comparison between the attraction indices of the species consumed by O. caerulescens sulferescens and their overall recovery rate.
Oedipoda caerulescens sulfurescens
Three plant species are obviously ingested by O. caerulescens sulferescens. They belong to the Lamiacea and Anacardiacea families. We also notice that the consumption rate remains closely related to the relative frequency of plant fragments found in the feces of O. caerulescens sulferescens.
We notice that Lavandula multifida is the most frequent species in the feces, therefore, the most consumed (F%=49.85%), followed by L. dentata (F%=45.57%), and subsequently by P. lentiscus, which seems to be the least ingested and the less attractive species at the same time (F%=4.61%; IA=0.38). Although the plant cover rate of L. multifida is the lowest (7.92%), it attracts more O. caerulescens sulfurescens because the attraction index is the highest among the three species (IA=6.57).
Discussion
In this work, we studied the diet and the trophic spectrum of O. miniata mauritanica and O. caerulescens sulphurescens in the Ghazaouet region (Tlemcen, Algeria); thus, we established a comparative analysis of the floristic composition of the plant cover of the biotope and the floristic composition of the feces of individuals captured in that same biotope. Thus, it was determined that these two locusts consume mainly Lamiaceae species and show a great polyphagia consuming at least three plant species; indeed, monophagous Orthoptera tend to be rare (Picaud et al., 2003). However, the different plant species present at the study site are not all part of the trophic spectrum of these locusts, for they show several degrees of selectivity in their diet.
Regarding the frequency of plant species in the feces of the two species, we noticed that each exhibits a food preference characterized by the choice of the ingested plants. This preference for one or more plants could be related to the accessibility of the plants accessibility or the nutritional needs throughout the year. According to Dajoz (1985). The choice of the plant is not due to its nutritional value or to its abundance in the field, but it seems that it may rather be linked to other stimuli influencing the feeding behavior of the locust (Otte et Joern, 1977; Launois-Luong, 1976). However, this trophic behavior cannot be fully understood if it is isolated from other activities, such as long distance trips or daily trips within a site, and this is because the food spectrum available to the locust changes. Wind tunnel experiments with Acrididae-from the Schistocerca genus-have shown the possibility of olfactory attraction over long distances (Dadd, 1963).
In addition, each phytophagous insect holds a complex system of chemical messages which trigger, regulate, and coordinate some of their feeding behavior sequences (Pesson, 1980). Their proliferation, in a given location, is better with the hypothesis of amelioration of trophic resources amelioration than with that of direct impact of climatic factors direct impact.
Many acidians bit unsuitable food plants before rejecting them (Clark, 1947; Pfadt, 1949; Johnston and Buxton, 1949; Williams, 1954). This act also demonstrates the importance of taste stimuli in food discrimination; nevertheless, there are cases where repulsive odors are encountered during orientation and before biting (Williams, 1954), food acceptability is probably determined primarily at this stage.
Dadd (1963) mentioned that there is no doubt that taste responses are involved in accepting (or rejecting) what is bitten and sustaining feeding afterward; further, hungry insects often bite with no other stimulus than contact with a substrate; indeed, they can attack unlikely materials (e.g. metals, glass, wax and wood) (Dadd, 1960), and it is quite conceivable that phytophagous insects, especially those that feed at the edge of the leaves, require only contact with a suitable surface to initiate feeding.
We noticed that O. miniata mauritanica shows a high affinity for the plant species L. dentata, whereas O. caerulescens sulfurescens manifests a high affinity for L. multifida. These results seem to agree with those of Mesli (2007), who reported that aromatic and medicinal plants form a major part of the locust diet, as locusts are attracted to fragrant plants. The latter author also confirmed that a locust can be qualified as euryphagous or stenophagous and that depending on the richness of vegetation richness in the environment. We also observed that plant species with low grassy cover; such as L. multifida; have a high attraction index and are sometimes overexploited by these locusts. In fact, of the 14 plant species present in the entire sampled area, the two Oedipodinae species consume only three plant species, translating into a trophic niche exploitation rate of only up to 21.42% for both species. Thus, we can deduce that they have the same trophic range in this region.
According to Chapman (1982) the majority of phytophagous insects have more than 50% of oligophagous or monophagous species, i.e., feeding on a single plant family; on the other hand, in Orthoptera and in particular in locusts, 60% of the species are polyphagous, and 25% are described as graminivorous. Picaud et al. (2003) mentioned that specialist Orthoptera species are scarce and that the graminivorous species choose their food according to the high sugar concentrations contained in the grasses; therefore, they have many food options when foraging. Indeed, Danoun (2016) studied the trophic spectrum of these two Oedipodinae in the mountains of Tlemcen (Ahfir region) and the highlands of the region of Tlemcen (Sebdou region) and observed that O. miniata mauritanica has a slightly wider trophic range than O. caerulescens sulfurescens and that despite their belonging to the same subfamily. The same author mentioned that these two species show polyphagia and eat mainly fatty (F. poaceae), thus joining Essakhi (2015) findings in the Morocco region. In fact, Isely (1944) indicates that graminivorous species have mandibles more adapted to the grinding of hard materials than those of forbivorous or mixed feeders and linked this morphological distinction to the fact that grasses are generally harder than the leafy parts of herbaceous plants.
According to Picaud et al. (2003), for grasphoppers, food appears to be selected on a nutritional basis likely to contain the high sugar content of grasses. This trophic preference for grasses was explained by Allal-Benfekih (2006) by the fact that grasses contain fewer molecules of secondary metabolism than dicotyledons. Several studies have shown that metabolites including alkaloids of the pyrrolizidines (Ben Halima, 1983), quinolizidines (Picaud et al., 2003), glucosides (Mainguet et Louveaux, 2000) or tannins (Boppré et Fischer, 1994) group are generally locust repellants.
Our study reveals that locust feeding behavior depends as much on its tolerances and requirements as on the abundance and quality of vegetation cover. Thus, the amplitude of feeding variation depends ultimately on the conditions prevailing in the environment, that is, climatic and edaphic factors can indirectly influence it and that by modifying the characteristics of the host plants, knowing that an insect can only feed if the plant has physical and chemical properties that suit its nutritional needs (Daget et Godron, 1984; Dajoz, 1985).
Besides, locusts seek food with low water content in humid environments, whereas they prefer water-rich food in dry ones (Millot, 1937), so, plants consumption, and thus, food choice can be induced by the environment. Therefore, the feeding behaviour of these insects through these aspects of food seeking, detection, and ingestion, then, constitutes an essential part of the insect/plant relationship.
Acknowledgement
We thank Mr. Bessenouci and Amir Khalil who supported me and helping me to make field trips. The authors thank Mr. Boukli Hacen for his valuable donations of material to for his laboratory and the great help in the identification of species. Special thanks also to Dekkak Soumya for helping to provide information regarding orthoptera.
References
Allal-Benfekih, L. (2006). Recherches quantitatives sur le criquet migrateur Locusta migratoria (Orth. Oedipodinae) dans le Sahara algérien.
Ben Halima, T. (1983). Etude expérimentale de la niche trophique de Dociostaurus marrocanus (Thunberg, 1815) en phase solitaire au Maroc, p:178.
Bennett, F.D. (1970). Insects attacking water hyacinth in the West Indies, British Honduras and the USA. Hyacinth Control Journal, 8:10-13.
Boppré, M., Fischer, O.W. (1994). Onocerus and chromalaena in West Africa. A chemeocological approach towards pest management, Eshborn, pp:107-126.
Butet, A. (1985). Méthode d’étude du régime alimentaire d’un rongeur polyphage (Apodemus sylvaticus L. 1758) par l’analyse microscopique des fèces. Mammalia, 49:445-483.
Chapman, R.F. (1982). Chemorereception: the signilcance of receptor numbers. Advances Insect Physiology, 16:247-356.
Clark, L.R. (1947). Ecological observations on the small plague grasshopper, Austroicetes cruciata (Sauss.) in the Trangie district, central western New South Wales.
Dadd, R.H. (1963). Feeding behaviour and nutrition in grasshoppers and locusts. In Advances in insect physiology. Academic Press. 1:47-109.
Dadd, R.H. (1960). The nutritional requirements of locusts. I. Development of synthetic diets and lipid requirements. Journalof Insect Physiology, 4:319-347.
Daget, P., et Godron, M. (1982). Analyse de l’écologie des espèces dans les communautés. Ed. Masson, Paris, p:163.
Dajoz, R. (1982). Précis d’écologie. Gautier-Villars. Paris, p:503.
Dajoz, R. (1985). Précis d'écologie. Bordas, Paris, p:505.
Damerdji, A., Mekkioui, A., et Doumadji-Mitiche, B. (2000). Mise en evidence d’Ampelodesmamauritanicum (diss) dans les fèces de différentes espèces de caelifères (Orthoptères) récoltées dans les monts de Tlemcen; Etude qualitative. Revue INRAA, 1:67-75.
Danoun, M. (2016). Bio-écologie et régime alimentaire des principales espèces d’Orthoptères dans la région de Tlemcen, p:137.
Doumandji-Mitiche, B., Doumandji, S., Benfekih, L. (1992). Preliminary data on the bioecology of the Moroccan grasshopper Dociostaurus maroccanus (Thunberg, 1815) in Ain Boucif (Médéa-Algeria). Mededelingen van de Faculteit Landbouwwetenschappen. Rijksuniversiteit Gent, 57:659-665.
Duranton, F., Launois, M., Launois-Luong, M.H., et Lecoq, M. (1982). Manuel de prospection antiacridienne en zone tropicale sèche. Gerdat (Ed), Paris. 2:696.
El Ghadraoui, L. (2002). Etudes bioécologiques de criquet marocain (Dociostaurus maroccanus) dans le site AL-Azghar du Moyen Atlas.
Essakhi, D. (2015). Contribution à l'étude du régime alimentaire des orthoptères acridiens dans le Moyen Atlas (Maroc). International Journal of Engineering and Science, 5:60-66.
Hochkirch, A., Gröning, J., Loos, T., Metzing, C., Reichelt, M. (2000). Specialized diet and feeding habits as key factors for the habitat requirements of the grasshopper species Tetrix subulata (Orthoptera: Tetrigidae). Entomologia Generalis, pp:39-51.
Isely, F.B. (1944). Correlation between mandibular morphology and food specificity in grasshoppers. Annals of the Entomological Society of America, 37:47-67.
Isely, F.B. (1946). Differential feeding in relation to local distribution of grasshoppers. Ecology, 27:128-138.
Johnston, H.B., Buxton, D.R. (1949). Field observations on locusts in eastern Africa. Anti-Locust Research Centre.
Kara, Z. (1997). Etude de quelques aspects écologiques et régime alimentaire de Schistocerca gregaria (Forkål, 1779) (Orthoptera, Cyrtacanthacridinae) dans la région d’Adrar et en conditions contrôlées. Alger, p:182.
Launois-Luong, M.H. (1975). Méthode d'étude dans la nature du régime alimentaire du criquet migrateur Locusta migratoria capita (Sauss). Annals Zoological and Ecological Animal, Paris.
Legal, P. (1989). T Le choix des plantes nourricières et la spécialisation trophique chez les acridiens (Orthoptera, Acrididae. Acrida, 8:2-8.
Louveaux, A. (1976). Prise de nourriture chez le criquet migrateur Locusta migratoria. Bulletin Social Zoology, France, 101:1052-1053.
Louveaux, A., Mainguet, A.M., et Gillon, Y. (1983). Recherche de la signification des différences en valeur nutritive observée entre feuilles de blé jeunes et âgées chez Locusta migratoria (R. et F.) (Orthoptera, Acrididae). Bulletin Social Zoology, France, 108:453-465.
Mainguet, A.M., Louveaux, A. (2000). Ability of a generalist insect, Schistocercagregaria, to overcome thioglucosidedefence in desert plants: tolerance or adaptation. Entomologia Experimentalis and Applicata. 94:309-317.
Mesli, L. (2007). Cointribution à l'étude bioecologique et regime alimenatire des principales espèces d'Orthoptères dans la wilaya de Tlemcen.
Millot, J., Fontaine, M. (1937). Etude physiologique sur les Orthoptères. La teneur en eau du criquet pèlerin adulte. Bulletin Social History Nature, France, pp:412-418.
Mohamed-Sahnoun, A. (1995). Bioécologie du peuplement Orthoptérologique de la station du col des Fougères (parc national de Chréa), p:158.
Otte, D., et Joern, A. (1977). On feeding pattern in desert grasshoppers and the evolution of specialized diets. Proceeding Academic Nature Science Philad, 128:89-126.
Pesson, P. (1980). A propos de l’Institut botanique des insectes: un aspect de la coévolution des plantes et des insectes. Annals Social Entomolgy, France, 16:435-452.
Pfadt, R.E. (1949). Food plants as factors in the ecology of the lesser migratory grasshopper, Melanoplus mexicannus (Sauss).
Picaud, F., Bonnet, E., Gloaguen, V., Petit, D. (2003). Decision making for food choice by grasshoppers (Orthoptera: Acrididae): comparison between a specialist species on a shrubby legume and three graminivorous species. Environmental Entomology, 32:680-688.
Prat, H. (1932). L'épiderme des graminées. Etude anatomique et systématique. Annals Science Nature, France, 10:329.
Williams, L.H. (1954). The feeding habits and food preferences of Acrididae and the factors which determine them. Transactions of the Royal Entomological Society of London, 105:423-454.
Zaim, A. (2013). Etude bioécologique des acridiens du Moyen Atlas, Perspectives de lutte biologique par les extraits des plantes.
Author Info
D. Meriem*, M. Lotfi and B. NadhiraCitation: Meriem, D., Lotfi, M., Nadhira, B. (2021). Diet of Oedipoda miniata mauritanica and Oedipoda coerulescens sulfurescens (Orthoptera: Acrididae) on the coast of the Tlemcen region (Algeria). Ukrainian Journal of Ecology 11 (8), 21-27.
Received: 06-Jul-2021 Accepted: 08-Oct-2021 Published: 23-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
