Research - (2021) Volume 0, Issue 0
Development of experimental production of algal biomass: case of Dunaliela salina of Arzew's Salines (Western Algeria)
B. Doukani, M.A. Bouzidi and A. Kerfouf*Abstract
This present work dealing with the study of the growth of a microalgae of economic interest, which proliferates in the salines of Arzew in western Algeria. Dunaliella salina is a microalgae endemic to these salty environments. Samples were collected and maintained as a pure culture of isolated Dunaliela salina. The aim of this study, is to establish a first reference state of the environments of the Salines of Arzew, and to find the best conditions for the development of this microalgae under experimental conditions. Two main ecological parameters (light and salinity) that influence the production of its biomass are studied. The site renowned for its richness in crustaceans of the genus Artemia salina and an important algal biomass, receives a large population of greater flamingo Phoenicopterus roseus. The water's salinity at the sampling site was 188 gr/l. The chlorophyll concentration and cell density were greater than 110 μg/l, and 2 × 106 cells/l respectively. The experimental results obtained demonstrated that the light intensity (18,000 lux) is the most influential parameter, followed by the number of days of algal culture. A cell concentration of 5.29 × 106 cells/l, is noted after 20 days of culture.
Keywords
Dunaliella salina, Microalgae, Ecological parameters, Biomass, Salines of Arzew, Western Algeria.
Introduction
Dunaliella salina, is a halotolerant unicellular chlorophyceae that lives in waters with a salinity of around 350 g/l (Borowitzka, 1990; Avron et Ben-Amotz, 1992; Leach et al., 1998; Krinsky, 2005). It can live in extreme conditions of salinity, temperature and solar radiation, due to the synthesis of a series of molecules that protect it (M. Ahmed et al., 2001; A. Bhatnagar et M. Bhatnagar, 2005), and currently recognized as the most salt tolerant eukaryote (Ben-Amotz et al., 1982; Loeblich, 1982; Garcia-Gonzalez et al., 2003; Gomez et al., 2003; Gomez et Gonzalez, 2005). D. salina is of biotechnological interest because it is capable of accumulating β-carotene, a pigment of natural origin, more active than that obtained by synthesis, used as a food coloring, source of vitamin A, and as an additive in cosmetology (Riahi, 2007). The objective of this work is to optimize the intensive production of microalgae biomass in experimental conditions, according to two ecological parameters: light intensity (I) and salinity (S).
Materials and Methods
Study area
The sampling site is a wetland classified by the Ramsar convention, for its rich flora and fauna, in particular migratory waterbirds. Sampling is carried out in the channel between the wetland and the salt basins for the production of salt (Fig. 1).
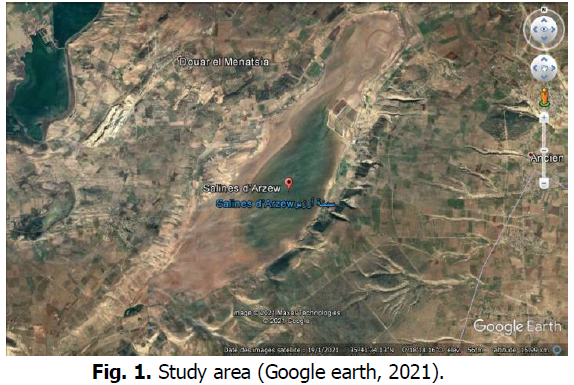
Figure 1: Study area (Google earth, 2021).
Sampling
A water sample is taken from the stagnant water channel that comes from the wetland (Fig. 2).

Figure 2: Sampling site.
The samples are cultured at low shaking, low light and at medium temperature. The color of the algae changes from red to green.
The identified pure algae samples are cultured in a place of low shaking, low light and medium temperature (Fig. 3A), until the color of the algae turns from red to green.
For each experiment, we carried out a pre-culture of Dunaliella salina with the same concentrations in a cylindrical reactor (Fig. 3B), in an air-conditioned hall, with a latest generation LED Day Light, and only atmospheric CO2. The temperature is kept constant at 25°C for all the experiments.
The sampled microalgae are cultured for 20 days in an aerated Johnson culture solution, and placed in a flat bioreactor (Fig. 3C): area (1 m2), thickness (40 mm) and volume (40 L).
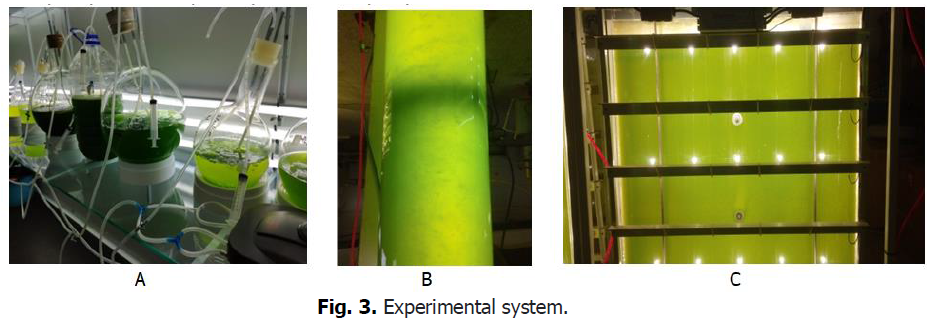
Figure 3: Experimental system.
Cell concentration is measured every 2 days using Thomas numbering cells. The design of the experiments makes it possible to simultaneously determine the individual and interactive effects of various factors that could influence the results (Box, 1951).
The objective of all the experiments is to evaluate the factors of effect on increasing the cell concentration of a microalgae in reactors in order to optimize the biomass yield. For these experiments, two parameters are chosen according to the bibliography: light intensity (I), and salinity (S). For each parameter, 4 progressive values are used for the light intensity (18000 lux, 28000 lux, 38000 lux and 45000 lux) and for the salinity (45 gr/l, 100 gr/l, 200 gr/l and 250 gr/the). Thus, 16 experiments are carried out, changing the various factors for each experiment (Table 1).
| Exp | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 18000 | 18000 | 18000 | 18000 | 28000 | 28000 | 28000 | 28000 | 38000 | 38000 | 38000 | 38000 | 45000 | 45000 | 4500 | 4500 |
| S | 45 | 100 | 200 | 250 | 45 | 100 | 200 | 250 | 45 | 100 | 200 | 250 | 45 | 100 | 200 | 250 |
Table 1. Parameter values for each experiment (Exp): light intensity (I: lux), and salinity (S: gr/l).
Results and Discussion
The water's salinity at the sampling site was 188 gr/l. The chlorophyll concentration in the sampling site and cell density were greater than 110 μg/l, and 2 × 106 cells/ml respectively.
Dunaliella salina microalgae are isolated and identified:
Phylum: Chlorophyta
Class: Chlorophyceae
Order: Chlamydomonadales
Family: Dunaliellaceae
Genus: Dunaliella
Species: Dunaliella salina (Dunal) (Téodor., 1905)
The color of algae sampled from the natural environment changes from red to green when cultivated at low agitation, low light and medium temperature (Fig. 4).
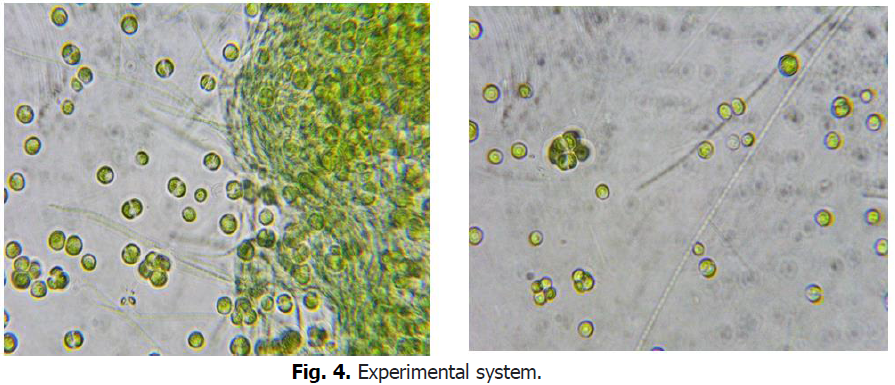
Figure 4: Experimental system.
For each of the 16 experiments carried out, the cell concentration is measured every two days, for twenty days (Table 2).
| Days/Experience | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.29 | 0.49 | 0.58 | 0.67 | 0.77 | 0.85 | 0.95 | 1.06 | 1.15 | 1.25 |
| 2 | 0.29 | 0.48 | 0.55 | 0.64 | 0.72 | 0.8 | 0.9 | 0.97 | 1.08 | 1.18 |
| 3 | 0.29 | 0.49 | 0.64 | 0.76 | 0.91 | 1.07 | 1.18 | 1.3 | 1.39 | 1.48 |
| 4 | 0.29 | 0.48 | 0.59 | 0.72 | 0.84 | 0.97 | 1.11 | 1.23 | 1.38 | 1.47 |
| 5 | 0.29 | 0.48 | 0.83 | 1.28 | 1.66 | 1.97 | 2.3 | 2.64 | 3.08 | 3.21 |
| 6 | 0.29 | 0.5 | 0.66 | 0.86 | 1.21 | 1.43 | 1.69 | 1.98 | 2.11 | 2.25 |
| 7 | 0.29 | 0.47 | 0.59 | 0.74 | 0.93 | 1.01 | 1.08 | 1.14 | 1.19 | 1.25 |
| 8 | 0.29 | 0.47 | 0.57 | 0.65 | 0.74 | 0.8 | 0.88 | 0.97 | 1.06 | 1.13 |
| 9 | 0.29 | 0.66 | 0.94 | 1.25 | 1.75 | 2.35 | 2.89 | 3.37 | 3.57 | 3.79 |
| 10 | 0.29 | 0.49 | 0.69 | 0.95 | 1.19 | 1.59 | 1.95 | 2.21 | 2.27 | 2.41 |
| 11 | 0.29 | 0.49 | 0.6 | 0.72 | 0.84 | 0.97 | 1.1 | 1.21 | 1.33 | 1.41 |
| 12 | 0.29 | 0.49 | 0.59 | 0.67 | 0.75 | 0.82 | 0.9 | 1 | 1.09 | 1.2 |
| 13 | 0.29 | 0.50 | 1.00 | 1.60 | 2.50 | 3.12 | 4.12 | 5.14 | 5.02 | 5.29 |
| 14 | 0.29 | 0.47 | 0.69 | 0.95 | 1.29 | 1.59 | 2.12 | 2.42 | 2.29 | 2.59 |
| 15 | 0.29 | 0.48 | 0.58 | 0.67 | 0.75 | 0.82 | 0.9 | 0.98 | 1.08 | 1.18 |
| 16 | 0.29 | 0.45 | 0.45 | 0.40 | 0.36 | 0.28 | 0.12 | 0.03 | 0.00 | 0.00 |
Table 2. Evolution of cell concentration (x 106/ml) compared to the number of days.
The results obtained from the cell counts of the cultures of Dunaliella salina, carried out every two days, demonstrate that this species of microalgae is capable of growing for all the salinities tested. The growth profile increases more with a salinity concentration of 45 gr/l, than that of 250 gr/l. By comparing the experiments for the same salinity (45 gr/l), the highest growth was observed on the twentieth day of the first experiment, for the lowest light intensity (45000 lux).
The growth of algae stops more with increasing salinity (250 gr/l), than at low salinity (45 to 100 gr/l). Ben-Amotz (1987), Dolapsakis et al. (2005), Oren (2005), Tawfik S Abu Rezk et al. (2010) found that optimal growth of Dunaliella can be achieved in coastal and lagoon and saline areas where the desired salt concentration can be achieved depending on natural weather conditions (precipitation and evaporation).
Experimental cultures 1, 2, 5, 6, 9, 10, 13 and 14 show that cell concentration changes with increasing salinity up to 100gr/l, but it decreases above this salt level. The same observation is noted for a light intensity of 38,000 lux.
Cellular concentrations obtained from D. salina grown at different light intensities (18000; 28000; 38000 and 45000 lux), demonstrated that algal growth also increases with increasing light intensity until inhibition of photosynthesis from 45,000 lux.
Dunaliella salina prefers high light intensity to low light intensity up to 5.29 x 106/ml cells at 45,000 lux and 45gr/l salinity and only up to 3.79 x 106/ml cells at 38,000 lux and 45 gr/l of salinity. These results are also consistent with the findings of Singh et al. (2000) who reported that Dunaliella salina increases at a much faster rate at high light intensities and medium salinity. Gomez et al. (1992) concluded that the rate of photosynthesis was significantly higher in green form at light intensities below 50,000 lux. However, photosynthetic inhibition in high light was more pronounced in the green form.
Richmond (1986), Borowitzka (1990), Renaud et al. (1991, 1995) noted that the chemical composition of many microalgae is influenced by growing conditions such as salinity, temperature, pH and nutrients. Leach et al. (1998) concluded that it was possible to obtain a cellular concentration of Dunaliella salina of 0.8x106 cells/ml when the culture is maintained at a salinity of 180 gr/l. The results obtained show that the light intensity is the most influential parameter, followed by the number of days of culture.
Conclusion
The optimal growth conditions for cell concentration deduced from the analysis of these experiments are: 18,000 lux for light intensity, 45 gr/l for salinity. The maximum was recorded at the end of the 20th day for a concentration of 5.29 x 106 cells/l.
Increasing cell concentration is a dynamic operation. Our experiments allow us to determine the importance of each factor as well as the interactions between them. To improve this study, we must perform a dynamic study that takes into account the growth rate, as a function of the time of incubation.
For the culture conditions of this study, it was possible to establish and maintain pure cultures of Dunaliella salina from the Salines of Arzew (western Algeria), in the laboratory. The results of the experiments carried out showed that this species preferred a high salinity of 45 gr/l for optimal growth.
Further test results showed that growth performance was limited for a concentration of 100gr/l in the green form, and that the growth performance for this strain was better at a high light intensity of 18000 lux at 45000 lux.
The values of the two parameters where growth is slowed are 45000 lux of light intensity and 200 gr/l of salinity. From these two values, the algae is constrained by osmotic pressure and light irradiation to concentrate β-carotene and glycerol to resist, which explains the slowing down of cell division and growth. however, algal biomass yields can be improved by optimizing other physicochemical production parameters.
References
Ahmed, M., Arakel, A., Hoey, D., Coleman, M. (2001). Integrated power, water and salt generation: A discussion paper. Desalination 134:37-45.
Avron, M., Ben-Amotz, A. (1992). Dunaliella: physiology, biochemistry and biotechnology. Boca Raton:CRC Press.
Ben-Amotz, A., Katz, A., Avron, M. (1982). Accumulation of β-carotene in halotolerant algae: Purification and characterisation of β-carotene-rich globules from Dunaliella bardawil (Chlorophyceae). Journal of Phycology, 18:529-537.
Bhatnagar, A., Bhatnagar, M. (2005). Microbial diversity in desert ecosystems. Current Science, 89:91-100.
Borowitzka, M.A. (1990). The mass culture of Dunaliella salina. Technical resource papers regional workshop on the culture and utilization of seaweeds. Regional Seafarming Development and Demonstration.
Box, G.E.P, Wilson, K.B. (1951). On the experimental attainment of optimum conditions. Journal of the Royal Statistical Society, Series B, 13:1-45.
Dolapsakis, N.P., Tafas, T., Abatzopoulos, T.J., Ziller, S., Economou-Amilli, A. (2005). Abundance and growth response of microalgae at Megalon Embolon solar saltworks in northern Greece: An aquaculture prospect. Journal of Applied Phycology 17:39-49.
Gomez, P.I., Barriga, A., Cifuentes, A.S., Gonzalez, M.A. (2003). Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC-30861) Chlorophyta. Journal of Biological Research, 36:185-192.
Gomez, P.I., Gonzalez, M.A. (2005). The effect of temperature and irradiance on the growth and carotenogenic capacity of seven strains of Dunaliella salina (Chlorophyta) cultivated under laboratory conditions. Journal of Biological Research, 38:151-162.
Garcia-Gonzalez, M., Moreno, J., Canavate, J.P., Anguis, V., Prieto, A., Manzano, C., Florencio, F.J., Guerrero, M.G. (2003). Conditions for openair outdoor culture of Dunaliella salina in southern Spain. Journal of Applied Phycology, 15:177-184.
Iltis, A. (1980). Les algues in. Durand JR et Leveque C, flore et faune aquatique de l’Afrique sahélosoudanaise. 1:873.
Krinsky, N.I, Johnson, E.J. (2005). Carotenoid action and their relation to health and disease. Molecular Aspects of Medicine, 26:459-516.
Leach, G., Oliveira, G., Morais, R. (1998). Production of a carotenoids-rich product by alginate entrapment and fluid-bed drying of Dunaliella salina. Journal of the Science of Food and Agriculture, 76:298-302.
Loeblich, L.A. (1982). Photosynthesis and pigments influenced by light intensity and salinity in the halophile Dunaliella salina (Chlorophyta). Journal of the Marine Biological Association of the United Kingdom, 62:493-508.
Renaud, S.M., Parry, D.L., Luong-Van, T., Kuo, C., Padovan, A., Sammy, N. (1991). Effect of light intensity on proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. Journal of Applied Phycology, 3:43-53.
Renaud, S.M., Zhou, H.C., Parry, D.L., Thinh, L.V., Woo, K.C. (1995). Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T.ISO). Journal of Applied Phycology, 7:595-602.
Riahi, J., Haouzine, Y., Akkal, R., Mouradi, A., Creach, A., Givereaud, T., Mouradi, A. (2007). Influence des nitrates, de la salinité et du stress lumineux sur la teneur en acide grs et en β carotène de Dunaliella salina. Bulletin Social Pharmacology of Bordeaux, 146:235-250.
Richmond, A. (1986). Microalgae of economic potential. In Handbook of microalgal mass cultures. Edited by A. Richmond. Florida: CRC Press, Inc., pp:199-243.
Singh, E., Babcock, R., Radway, J.A. (2000). Photobioreactor modification for Dunaliella salina. Marbec Summer Undergraduate Research Fellowship, Marine Bioproducts Engineering Center, University of Hawaii at Manoa and University of California, Berkeley.
Tawfiq, S.A., Al-Hooti, S., Jacob, D.A. (2010). Optimum culture conditions required for the locally isolated Dunaliella salina. Journal of Algal Biomass Utln, 1:12-19.
Author Info
B. Doukani, M.A. Bouzidi and A. Kerfouf*Citation: Doukani, B., Bouzidi, M.A., Kerfouf, A. (2021). Ecological assessment of variability of quantitative signs of spring wheat samples. Ukrainian Journal of Ecology, 11 (8), 167-171.
Received: 29-Sep-2021 Accepted: 23-Oct-2021 Published: 25-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
