Research Article - (2022) Volume 12, Issue 3
Contribution to the knoweldge of earthworm fauna of Chrea National Park (Algeria)
H. Zerrouki*, S. Hamil, M. Alili, W. Isserhane and M. BahaAbstract
This preliminary study was conducted to identify the different species of earthworms that live in the region of Chréa in north Algeria. A total of 3196 earthworms were sampled and represented by three families: Acanthodrilidae, Megascolecidae, and Lumbricidae. Eleven genera represented by nineteen species are discovered. The individuals were identified to the following taxa: Aporrectodea caliginosa, Allolobophora rosea, Allolobophora cholorotica, Allolobophora antipai, Allolobophora miniscula, Aporrectodea trapezoides, Eiseniella tetraedra, Amynthas sp., Amynthas californica, Microscolex phosphoreus, Criodrilus lacuum, Octodrilus complanatus, Octodrilus maghrebinus triginta, Octodrilus maghribinus maghribinus, Eisenia xylophila, Ocnerodrilus sp, Eisenia andrei, Helodrilus oculatus, Dendrobaena pantaleoni. D. pantaleoni is new record for Algeria. High abundance of earthworms have been recorded under the cork oak, where the soil is rich in organic matter, followed by cedar soils that are characterized by lower organic matter content. Both species A. caliginosa and O. complanatus are absent in a soil poor of organic matter and under Quercus canariensis.
Keywords
Earthworms fauna, Soil, Diversity, Chrea national park.
Introduction
For several decades, the study of soil's living world has become increasingly important. Among these organisms, earthworms have always aroused the most interest. Aristotle was one of the first people who draw attention to the role of earthworms in turning over the soil. In his last work, Charles Darwin (1881) drew the attention of the scientific world to earthworms. Several researchers then became interested in physiology, general biology, their participation in soil fertility, and their pedogenetic activities as (Hensen, 1877; Bornebusch, 1930; Laverack, 1963 and others to date.
For the taxonomic purpose, first works came from Cuvier and Lamarck whose recognized the class of Annelids (Lamarck, 1800). Important studies were subsequently published by: Savigny 1826; Rosa, 1893; Michaelsen,1906; Tetry, 1937; Zajonc, 1959; Graff, 1962; Bouché, 1972; and Omodeo, 1987. Earthworms in the Maghreb had been little studied and the knowledge of their diversity is limited and incomplete. The first collection of earthworms in Maghreb was carried out in Egypt. These studies started with Napoleon’s expedition of 1798 and his attempts to record Egypt’s natural history. The (few) worms collected were described by Savigny (1809), but do not fit any identifiable species.
Establishment of protected areas is a key strategy in biodiversity conservation (Vale, et al., 2018), but at the same time, it is primarily driven by available opportunities rather than scientific knowledge (Baldi, et al., 2017). In this regard, it is very important to study biodiversity on a local scale in order to provide the best possible management options.
Earthworms are essential service providers for terrestrial ecosystems (Lavelle, et al., 2006). Their activity, generating galleries and casts, contributes to formation and maintenance of soil structure (Lavelle, 1997; Capowiez, et al., 2012), increasing porosity, infiltration and water retention (Fiuza, et al., 2012), as well as re-distribution and breakdown of soil organic matter (Brown, et al., 2000). However, earthworms are sensitive to land use and management, and can be used as soil quality and management as well as environmental bioindicators (Brown and Domínguez, 2010; Bartz, et al., 2013; Bünemann, et al., 2018). Algeria is home to more than 31 described earthworm species (Zeriri, et al., 2013; Baha and Isserhane, 2017), but practically nothing is known of the species and populations inhabiting protected area in the country.
The earthworms of Algeria started receiving attention in the second half of the past century Beddard (1892), Rosa (1893). A few additional records were published in the first decades of this century Michaelsen (1903-1930); (Bouché, 1972). However, these older records were scanty and all came from occasional collections.
The first extensive survey was made by Omodeo and Martinucci (1987), who found 24 species, of which three were new to science and 14 were new for Algeria. BAHA (1997) presented a collection of 500 earthworms taken during (1990-1991) from various soils under orange cultivation in Mitidja, a coastal plain to the south of Algiers. Of the 11 species identified, Allolobophora chlorotica Savigny (1826) was new for North Africa, other three species had not yet been reported from Algeria, and a fifth Prosellodrilus doumandjii Baha and Berra 2001, was shortly to be described as new to science.
During that study a variety of habitats were prospected, but no sampling was made in cultivate areas. In this paper were stadied a collection of earthworms taken during (2015-2018) from different soils under different vegetation in Chrea National Park (Algeria). It emerges of all these studies more than 8000 species from about 800 genera in the world (Edward, 2004). In Algeria Twelve genera, represented by 31 species (Zeriri, et al., 2013; Baha and Isserhane, 2017), have been revealed. Earthworms are common all over the world in natural forests and grasslands as well as agrosystems and found in most regions of the world.
However, almost no data on earthworms are available from the northern part of Algeria (Chréa National Park). The present study aimed to investigate earthworms’ diversity and spatial distribution and abundance in the Chréa Biosphere Reserve and its vicinity, situated in the north center of Algeria, and give the first systematic data on earthworms from this region.
The main objective of the present research was to gain a piece of scientific information about kind of earthworms, their relative abundance, seasonal fluctuations of population and evaluate earthworm communities (abundance, biomass, species composition) associated with different forest habitats of Chréa Biosphere Reserve. Such information could be used to suggest measures for the improvement of soil to increase forest land production.
Materials and Methods
Study area
This research was carried out in the Chrea National Park. It is located in central area of Algeria (36°19’-36°30’N and 2°38’-3°02’E), about 50 km south-west of Algiers (Fig. 1). It covers around 26 587 ha over a length of 40 km from E/W and a width of 7 to 14 km from N/S (Meddour, 1994); of higher mountains (900-1550 m) in the Mitidja area. The climate is fresh and humid, with a mean annual rainfall of 950-1200 mm, monthly mean of temperatures between 3-7°C in winter and 18-23°C in summer; snow is relatively frequent (50-100 cm annually) (Sbabdji, 2012).
Fig 1: Geographical location of the study site within Chrea National Park (PNC).
Earthworms sampling and laboratory analyses
Earthworms were sampled during the four seasons from September 2014 to June 2017. The six sampled sites are between 36°19'-36°30'N and 2°38'-3°02'E, their respective altitude, latitude, longitude, type of vegetation and the bioclimat are listed in Table 1. Sampling was made according to the criteria suggested by Lavelle (1988) and Anderson and Ingram (1993): at each site 10 samples of 25 × 25 × 30 cm were taken at regular 5 m intervals, along a line whose origin and direction had been randomly chosen. Each sample was given a code, and preserved worms were then transferred to labelled specimen bottles. They were preserved in 10% formalin and then transferred to 70% alcohol for permanent fixation. Only a minimum number of worms was preserved from each study site, and others were released back into the soil. Identification of the species was principally based on Bouche's descriptions, 1972; Omodeo, 1987; Sims, 1972; Tetry, 1937; Lee, 1985.
| Sites Characteristics | Col des Fougères (CF) | Forêt noire (FN) | Hakou Ferraoun (HF) | Sidi Rabah (SR) |
Dhaia (DH) |
Hammam Melouane (ML) | |
|---|---|---|---|---|---|---|---|
| Altitude | 1541 | 1333 | 750 | 500 | 1230 | 373 | |
| Latitude | 36°25’45.0”N | 36°25’08”N | 36°27’09.1”N | 36°22’04.3”N | 36°21’58.33”N | 36°25’43.4”N | |
| UTM | 489725.6583 | 490097.8583 | 487685.0466 | 490662.0976 | 472312.3169 | 497307.6452 | |
| Longitude | 02°53’10.9”E | 02°53’25”E | 02°51’47.9”E | 02°53’47.9”E | 02°41’31.56”E | 02°58’43.4”E | |
| UTM | 4031569.4260 | 403429.0050 | 4034163.2530 | 4024768.5062 | 4024623.5744 | 4031514.5142 | |
| Slope (%) | 10 | 5 | 10 | 15 | 10 | 10 | |
| Vegetation | Stratum herbaceum | Sedum villosum Senecio vulgaris Paranychia argenea Lamium Erodium cicani |
Satureja vulgaris Orchidée Sedum villosum |
Galium rotundifolium Rubus ulmifolium Geranium robertianum Tamus communis Andryala integrifolia Campanula ranunculus |
Geranium robertianum Galium rotundifolium Galactites tomentosa Andryala integrifolia Quercus suber Lodesma mauritanica |
Origanum glandulosum Sinapis pubescens Fedia cornucopiae Geranium robertianum |
Galactites mentosa Andryala integrifolia Campanula ranunculus |
| Shrub layer | Citrus villusus Juniperus oxycedrus |
Juniperus oxycedrus | Cistus triflorus Rosa carina Rhamnus alternus |
Pistacia lentiscus Rosa carina |
Juniperus oxycedrus Cystus villosus |
Rosa carina Cistus triflorus |
|
| Tree layer | Cedrus atlantica | Cedrus atlantica | Quercus canariensis Cedrus atlantica Quercus ilex |
Quercus suber Quercus ilex |
Quercus ilex Quercus faganae Clematis flammula Crataegus monogyna Prunus avium |
Pinus alepensis Quercus ilex |
|
| Bioclimatic stage | Sub humide à hiver frais Sub Wet in cool winter |
Humide à hiver froid Wet in cold winter |
Sub humide à hiver doux Sub wet in mild winter |
||||
Table 1. Sampling sites and their characteristics in Chrea National Park (September 2014 to June 2017).
Diversity analysis: In order to exploit the results obtained from ecological indices such as the Specific richness SR (SR=Sp1+Sp2+………+Spn) is the total number of species observed during N surveys, the Shannon-Weaver diversity (H'), H’=-Σ(ni∕N) log2 (ni∕N), Shannon and Weaver (1963) corresponds to the calculation of the entropy applied to a community, Clarke and Warwick (2001) and Margalef index (Dmg) (MARGALEF, 1958), Dmg=(S-1)/ln N; where pi=ni/N, ni=the average density of species, N=the average density of individuals in the sample, and S=number of species. Here, the event categories will therefore be represented for the species and their probability of occurrence pi by the ratio of the number of units of each of them nor to the total number of individuals present in the community N the maximum diversity (H'max) diversity is maximum when all the species in the stand would be represented by the same number of individuals. and equity (E) are used. fairness E varies between 0 and 1, Shannon and Weaver (1963). It tends towards 0 when almost all the numbers correspond to a single species in the stand and tends towards 1 when each species is represented by a similar number of individuals. The value of the Shannon-Weaver diversity index usually ranges from 1.5 to 3.5, only rarely exceeds 4.5, equity Equality Index (E) It expresses the degree of equality in species abundance in the sample, are used.
Soil analysis
Soil was collected from each region and divided into subsets for analysis. Soil texture was determined using the rapid method (Kettler, et al., 2001). To determine soil moisture, the soil was dried at 105°C for 48h. CE (Electrical Conductivity) and pH were measured using a digital meter. Soil N was determined by Kjeldahl technique (Misra, 1968). Organic C was determined using air dried and sieved soils samples using the wet oxidation method.
Statistical analysis
Different statistical analysis were carried out to exploit our results and to study the effect of numerous factors on the earthworms’ repartition, we have chosen the Factorial Analysis of Correspondences (AFC) analysis, have been realized with the use of PAST v 1.9. mustache boxes and ecological index graphs are produced by R programme (R version 3.4.4; R Development Core Team 2018).
Results
Soil characteristics
The results of the physico-chemical properties are shown on Table 2. The texture range from silty clay to silty sand. The granulometric analyses of the soil samples showed that the highest percentage of sand (61.17%) recorded was in the Dhaia while the lowest percentage (25.20%) was registered in the Col des Fougeres. Soil pH was slightly neutral in the majority of soil samples. The maximum and minimun soil moisture was 48.82% and 2.46% in Foret Noire, Dhaia respectively. The rate of organic matter is medium to high, it varies between 1.58 and 2.26%. The total nitrogen rate varies between 0.009 and 0.019%, with an increasing value in the Dhaia station. The CE is between 0.3 and 0.82 µS/cm; results indicate unsalted soils. For the other elements, the variation ranges for:P (141.75-160.16 ppm), K+ (0.43-0.5 mEq/100 g), Ca++ (8.8-9.96 mEq/100 g), C.E.C. (16.49-17.4 mEq/100 g), Na+ (3.15-4.2 mEq/100g) and Mg++ (1.38-2.63 mEq/100 g).
| Sites | Chrea sector | El Hamdania sector | Hammam Melouane sector | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | CF | FN | HF | SR | DH | ML | |
| Texture | Silty Clay | Silty | Silty sand | Silty Clay | Silty sand | Silty Clay | |
| Soil type | Poorly evolved | ||||||
| Granulometry | A | 34.93 | 23.9 | 26.1 | 29.7 | 12.25 | 31.2 |
| LF | 23 | 19.87 | 20.17 | 19.97 | 30.75 | 21.17 | |
| LG | 16.87 | 18.83 | 17.4 | 17.6 | 16.2 | 18.3 | |
| SF | 3.93 | 3.27 | 5.27 | 5.27 | 4.17 | 7.27 | |
| SG | 21.27 | 34.13 | 31.07 | 27.47 | 57 | 22.07 | |
| pH | 7.08 | 6.88 | 7.44 | 6.25 | 7.4 | 6.58 | |
| CE 25° ms/cm | 0.65 | 0.82 | 0.56 | 0.43 | 0.3 | 0.58 | |
| Humidity (%) | 27 | 48.82 | 17.7 | 28.72 | 2.46 | 33.4 | |
| Organic carbon % | 1.03 | 1.07 | 0.79 | 0.89 | 1.2 | 1.13 | |
| Organic matter % | 2.06 | 2.14 | 1.58 | 1.78 | 2.06 | 2.26 | |
| Total Nitrogen % | 0.011 | 0.019 | 0.01 | 0.009 | 0.002 | 0.015 | |
| Assimilable phosphorus P2O5 (ppm) | 145.95 | 152.3 | 141.75 | 142.3 | 160.16 | 153.17 | |
| Exchangeable K+ mEq/100g of soil |
0.47 | 0.44 | 0.46 | 0.5 | 0.44 | 0.43 | |
| Exchangeable Ca++ mEq/100 g of soil | 9.5 | 9.35 | 9.77 | 9.96 | 9.7 | 8.8 | |
| Exchangeable Na+ mEq/100 g of soil | 3.57 | 3.75 | 3.15 | 4.2 | 4.1 | 3.9 | |
| Exchangeable Mg++ mEq/100 g of soil | 2.05 | 1.65 | 2.63 | 1.38 | 2.2 | 2.56 | |
| Cation exchange capacity C.E.C mEq/100 g | 16.89 | 16.49 | 17.35 | 17.4 | 17.2 | 17.08 | |
Table 2. Physico-chemical analysis of the soil collected from different study sites in National Park of Chrea.
Oligochaetes
Nineteen earthworm taxa have been recorded in Chrea National Park belonging to three families (Table 3). Acanthodrilidae had the lowest diversity of species, with only one species. In contrast, Lumbricidae had the greatest diversity with 16 identified taxa .
| S.No | Code | Species name | Freq. (%) | Dom. (%) | Seasons distribution | Station. |
|---|---|---|---|---|---|---|
| 1 | Mph | Acanthodrilidae Microscolex phosphoreus (Dugès, 1837) |
25 | 2.19 2.19 |
A, Sp | CF, SR, HF and FN |
| 2 | Ams | Megascolicidae Amynthas sp. |
29.16 | 3.31 1.43 |
A, Sp, W | SR, HF and DH |
| 3 | Amc | Amynthas californica (Kinberg, 1867) | 25 | 1.87 | all Seasons | SR, HF and ML |
| 4 | Crl | Lumbricidae Criodrilus lacuum (Hoffmeister, 1845) |
41.66 | 94.39 1.78 |
all Seasons | SR, HF and ML |
| 5 | Eit | Eiseniella tetraedra (Savigny, 1826) | 29.16 | 1.68 | all Seasons | SR, HF and DH |
| 6 | Ocm | Octodrilus complanatus (Duges, 1828) | 70.83 | 21.50 | all Seasons | CF, SR, HF, FN and ML |
| 7 | Omt | Octodrilus maghribinus triginta, (Omodeo and Martinucci, 1983) | 66.66 | 7.23 | all Seasons | CF, SR, HF, FN and DH |
| 8 | Omm | Octodrilus maghribinus maghribinus (Omodeo and Martinucci, 1983) | 58.33 | 6.16 | all Seasons | CF, SR, HF and FN |
| 9 | Apc | Aporrectodea caliginosa (Savigny 1826) | 83.33 | 11.61 | all Seasons | CF, SR, HF, FN and ML |
| 10 | Apt | Aporrectodea trapezoides (Savigny, 1826) | 20.83 | 9.95 | A, Sp | SR, HF, FN and ML |
| 11 | Eix | Eisenia xylophila Omodeo and Martinucci 1987 | 16.66 | 0.47 | all Seasons | DH |
| 12 | Alr | Allolobophora rosea (Savigny 1826) | 83.33 | 16.86 | all Seasons | CF, SR, HF, FN and ML |
| 13 | Ala | Allolobophora antipai (Michaelsen, 1891) | 25 | 2.47 | all Seasons | ML and DH |
| 14 | Alm | Allolobophora miniscula (Rosa, 1905) | 29.16 | 1.94 | all Seasons | HF, FN and ML |
| 15 | Alc | Allolobophora chlorotica (Savigny, 1826) | 37.50 | 5.63 | all Seasons | SR, HF and ML |
| 16 | Odr | Ocnerodrilus Sp | 29.16 | 2.69 | A, Sp, W | CF, SR, HF and FN |
| 17 | Eia | Eisenia andrei (Bouché, 1972) | 29.16 | 1.03 | A, Sp, W | CF, SR and HF |
| 18 | Hlo | Helodrilus oculatus (Hoffmeister, 1845) | 8.33 | 2.69 | A, Sp | CF |
| 19 | Del | Dendrobaena lusitana (Graff, 1957) | 12.50 | 0.78 | A, Sp | CF, SR and FN |
Table 3. Species composition, frequency, and distribution of the Oligochaetes assemblage in National Park of Chrea.
The analysis of the total collection at Chrea National Park indicated the dominates of, the Lumbricidae family with 94.39% of individuals and 16 taxa. O. complanatus and A. rosea appear by far the most common and widespread taxa. They represent 21.50% and 16.86% of the specimens collected, they are found from east to west throughout the entire region (respectively 71% and 83% of the localities). The two families Acanthodrilidae and Megascolicidae had minor contributions (2.19 to 3.31% of individuals, respectively), and are not recorded in one of the six stations (ML).
Fig. 2 shows that the study sites seems to play an important role in the abundance distribution of earthworm. We have noticed that CF and HF are characterized by variable abundances. In CF station A. rosea (362 ind/m2) represents the maximum abundance, but M. phosphoreus 19 ind/m2 constitutes the minimum abundance. The existence of existence of O. complanatus was observed in the HF sation with a max abundance (478 ind/m2) whereas the minimum one is represented by C. lacuum (11 ind/m2). However, in the DH site we notice only low abundances. For the other sites (ML, NF, SR) we remark an average interval.
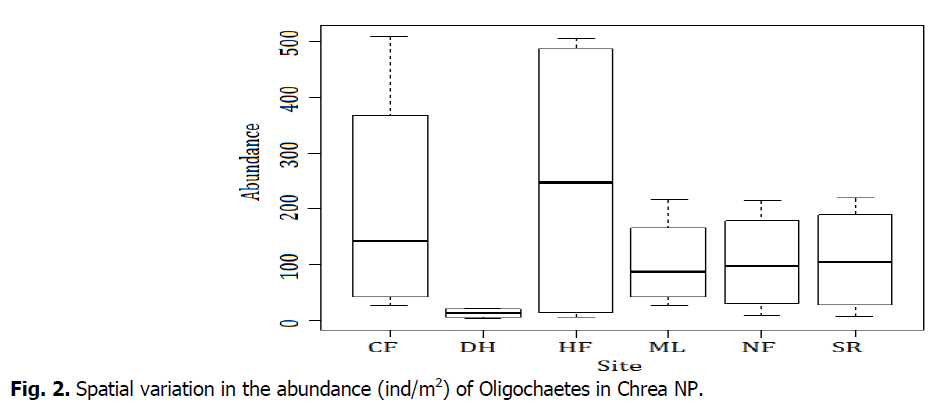
Fig 2: Spatial variation in the abundance (ind/m2) of Oligochaetes in Chrea NP.
The Fig. 3 shows that the season factor seems to play an important in the repartition of earthworm species. Autumn is characterized by variable abundances. In fact, we have observed existence of the species in big abundances, it is the case of O. complanatus (519 ind/m2) and A. rosea (509 ind/m2), but also low abundances concerning O. maghribinus maghribinus (117 ind/m2). All the species are low abundance in winter and summer. There are two atypical abundances for the spring group O. complanatus species with abundance 478, D. lusitana 19.
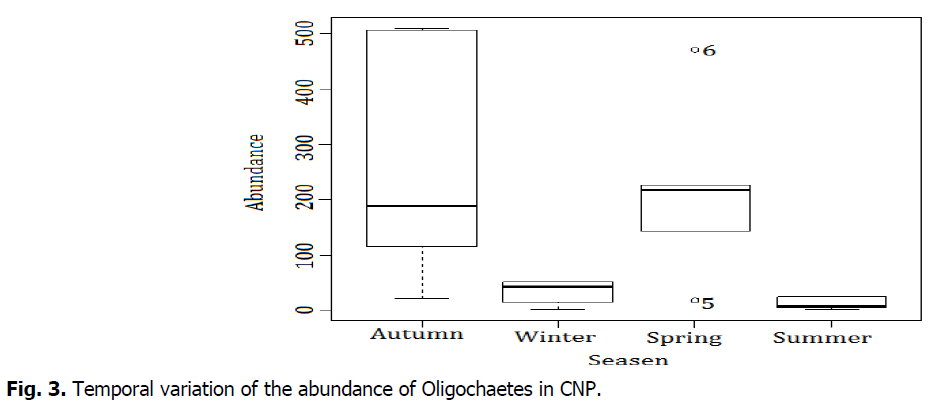
Fig 3: Temporal variation of the abundance of Oligochaetes in CNP.
The results indicate a significant fluctuation in the Shannon-Weaver H Diversity Index and E Equality Index in function of the seasons and study sites. Referring to the Shannon-Weaver H and Equality Index values, the results show a great diversity of species in spring, fall and winter seasons compared to summer. In addition, the most important diversity is observed in two sites Sidi Rabeh and Hakou Ferraoun, compared to the other study sites. The Fig. 4 specific richness SR, margalef index (Wealth Index) Dmg hows that, the highest abundance values are reported in periods of spring and fall, while the lowest are recorded in summer and winter seasons. The comparison of abundances according to study sites shows that, the highest abundance is reported in Hakou Ferraoun and Col des Fougères by compared with other sites.
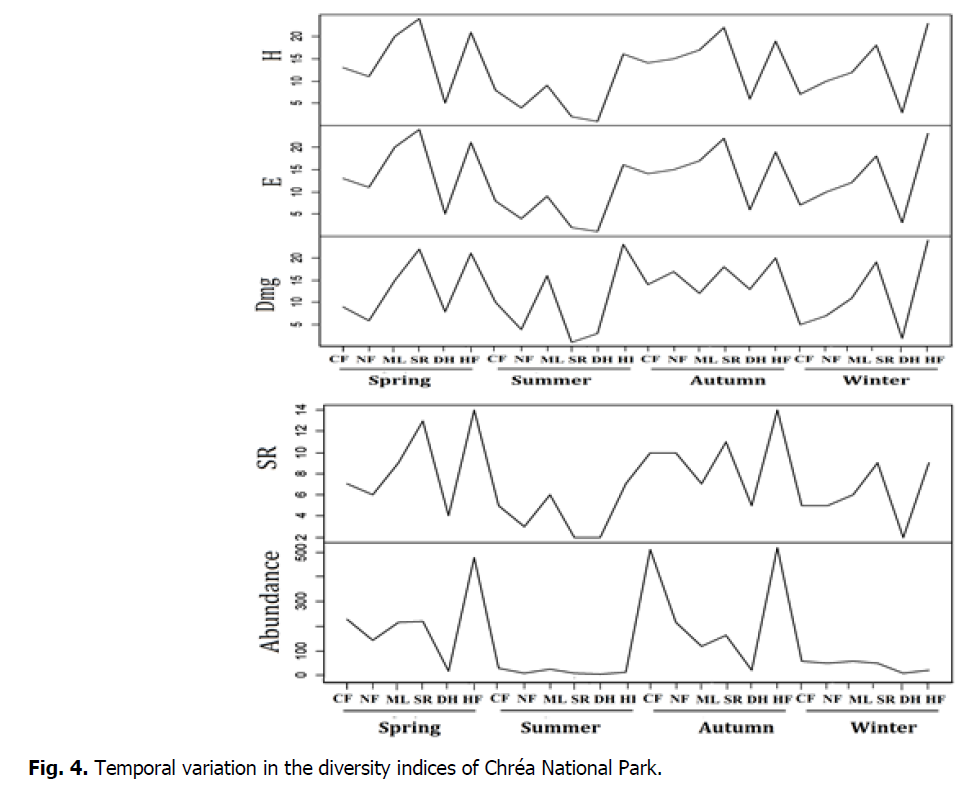
Fig 4: Temporal variation in the diversity indices of Chréa National Park.
Analysis of Oligochaete species from different stations Chréa Natinal Park
Three groupes of olighochaete species were found when changing the station, the latter are clearly visible within the dendrogram of the ascending hierarchical classification on the basis of a similarity of -3.2 (Fig. 5).
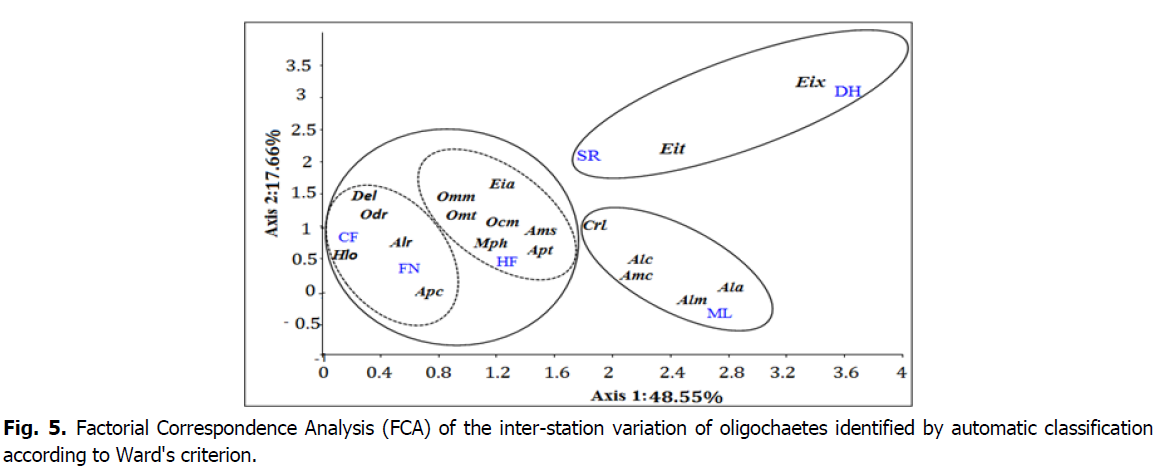
Fig 5: Factorial Correspondence Analysis (FCA) of the inter-station variation of oligochaetes identified by automatic classification according to Ward's criterion.
So as to exploit the results through statistical analysis methods and explain distribution of earthworms according to several factors, we have chosen the Analysis of Factor Analysis of Correspondences (AFC)The AFC was followed by automatic classifications on the first factorial coordinates using the Ward's grouping criterion. by the software PAST v 1.9.
The first group is represented by the species in the Dhaia and Sidi Rabah stations, this group is composed by E. tetraedra and E. xylophila.
The second group is represented by the species in the Magtaa Lazrag station, this group is composed of C. lacuumn, A. californica, A. chlorotica, A. antipai, A. miniscula.
The third group is represented by two subgroups, the first subgroup is composed of the species in the Col des fougères and Foret noir stations, this group is composed by D. lusitana, Ocnerodrilus Sp, A. rosea, H. oculatus, A. caliginosa. The second subgroup is composed of the species in the Hakou Ferraoun station this subgroup is composed of O. maghribinus maghribinus, O. maghribinus triginta, O. complanatus, A. trapezoides, E. andrei, Amynthas sp., M. phosphoreus.
Discussion
Nineteen species belonging to Eleven genera and three families have been identified: Acanthodrilidae (Dom, 2.19%), Megascolecidae (Dom, 3.31%), and Lumbricidae (Dom, 94.39%). The individuals were identified to the following taxa: A. caliginosa, A. rosea, A. cholorotica, A. antipai, A. miniscula, A. trapezoides, E. tetraedra, Amynthas sp, A. californica, M. phosphoreus, C. lacuum, O. complanatus, O. maghrebinus triginta, O. maghribinus maghribinus, E. xylophila, Ocnerodrilus sp, E. andrei, H. oculatus, D. pantaleoni.
Earthworms are one of the most diversified groups of soil fauna; these species vary mainly according to the physical and chemical properties of the soil. Their numbers fluctuate randomly every year depending on environmental conditions and biological interactions with other organisms. Temperatures, humidity and soil factors are key regulators of the abundance and activity of earthworms in the wild. Earthworm populations respond relatively quickly to changes of these environmental factors (Sims and Gerard, 1999). According to Bachelier (1963), their number fluctuates randomly every year depending on environmental conditions.
Humidity affects the spatial distribution of worms, it varies dynamics of populations (Edward and Bohlin, 1996) positively in high humidity, negatively in the case of low humidity. Resistance to drought as well as to water excess vary according to species and regions. We note that in four stations O. complanatus is the predominant species because humidity is high. But if we refer to the DH station, where humidity is low, its abundance is low (5 ind/m2). We note that in these five stations, the presence of endogeic earthworms is eminent according to (Johnston, et al., 2017), this group supports well large variations. In contrast to sensitive anecics whose presence is low.
When the soil dries up, earthworms, if they do not die, go deep in the soil, wrap as a ball and partially dry. They lose up to half of their water; their inactivity ceases with the return of water and their re-imbibition was diapause (Bouché, 1972). Analyzing our samples, we found that some species returned to Diapause, other types dug in depth, especially in dry seasons, while others were spread near the presence of water, this explains the difference in environmental groups. They prefer oak leaves, drop needles of conifers, and avoid herbs with thick and narrow roots (Bachelier, 1972). What we noticed in our work is that they dominate under oak in SR, ML and HF terminals, while in DH station with conifer dominance is minimal.
Omodeo, et al. (2003) believe that earthworm biodiversity is low over the whole Maghreb area (Morocco, Algeria and Tunisia). They identified 33 species, of which 24 taxa were collected in Algeria. Baha (1997) has identified 11 species in the Mitidja Algiers area. In our works, we have identified 19 studied species belonging to the Megascolecidae, Lumbricidae and Acanthodrilidae families. Earthworms are generally well-known in humid Europe and to a lesser extent in the Mediterranean countries of western Europe (Bouche, 1972). This fauna is probably similar to that of North Africa (Omodeo and Martinucci, 1987; Bouché, 2003). The three worm families have been observed in six stations of Chréa National Park; small and large tribal areas (Omodeo and Martinucci, 1987); in northern Algeria (Omodeo, et al., 2003); in eastern Algeria (Zeriri, et al., 2013).
Worms are distributed according to pH; according to Bouche's study (1972), the species are distributed with respect to soil pH and distinguishes between acidophils (pH<6), neutrophils (6<pH<7) and basophils (pH>7). In 1965, El-Duweini and Ghabbour reported little information about the distribution of earthworms according to the pH. Satchell's work (1955) distinguished the acid-tolerant animals, living in litter and those "acid-intolerant" digging the ground. Basic soils with a pH above 10 are unfavorable for worms (Lavelle and Spain, 1995). We note that most stations have a pH of 6.5 to 7.5. In general, most species are likely to be encountered in soils with a pH higher than 7. However, some species such as C. lacuum, E. andrei and E. tetraedra have been found in soils at pH 6.25-6.5; note that A. caliginosa is present in soils with a pH ranging from 6.25 to 7.5.
Salinity increases in dry soils because they are poor in organic matter. Earthworms cannot survive in this type of soils that are characterized by high or medium salinity. Most of the studied stations are less salty except for the DH station. Logically, in DH station the numbers of worms are very low.
Abundance or not Organic matter also plys a key role in distribution of earthworm. Earthworm populations feed on more or less decomposed Organic matter, on the surface or in the soil. It is the main food resource, and thus, this has considerable effect on the populations of earthworms. In most ecosystems, insects of soils are a key factor in the fragmentation of organic matter (Lavelle and Spain, 1995). It is thus estimated that they are able to consume almost all of annual litter (Brown, et al., 2002). The obtained results in our work show that soils of the six selected stations of the PNC are considered to be rich in organic matter. These stations representing a high rate of organic matter reveal a low abundance of the earthworms. This is indeed due to the effect of anthropization that some species do not support (Lavelle and Spain, 2001). This highly degraded organic matter will continue its cycle by being ingested by the earthenware, it undergoes then a new process of degradation and dissolution. This joint activity of earthworms and thus, contributes to the entry of carbon into soils and will allow all physical, chemical and biological processes.
Distribution according to the carbon/nitrogen ratio. The C/N ratio is one of the most explanatory elements in the distribution of Earthworms; it has a high biological significance (Bouché, 1972). The distribution study of the C/N distributions in which these species are found makes it possible to characterize three types of taxa. Eubiotics are major consumers favoring aerobic phenomena and soil structure; C/N=13. The forms living in at C/N>13, are qualified as mesobiotics. The extreme case, C/N>17, includes species from very specific environments. Note that the results obtained show a variable nitrogen content from one station to another. The DH station has a high nitrogen content; this can be explained by the presence of a category of earthworm (anecic) such as A. chlorotica which rejects the turricules on the soil surface. These biostructures are thus generally characterized by higher mineral content than in the surrounding soil. In particular, the contents of mineral nitrogen phosphorus are highly available in earth turricules (Blanchart and Brauman, 2010). The first two groups are distinguished in the PNC. The mesobiotics group includes: M. phosphoreus and C. lacuum. The species belonging to the eubiotics group are: M. phosphoreus, E. tetraedra, O. complanatus, O. maghribinus triginta, O. maghribinus maghribinus, A.caliginosa, A. rosea, A. chlorotica. This group is spread in CF, FN, SR and HF, but it is rare in DH and ML.
Distribution by Phosphorus; the obtained results showed that DH station is characterized by a high level of phosphorus, due to the presence of species like E. tetrahedra and E. xlylophila, terricules of earthworms and the abundance of endogeous species. These terricules are characterized by a higher P content (Kuczak, et al., 2006; Chapuis-Lardy, et al., 2011). However, the increase in P also concerns organic P and microbial P (Guggenberger, et al., 1996; Jimenez, et al., 2003). However, Zhang, et al. (2000) observed an increase in inorganic P in the soil in presence of earthworms.
Conclusion
A systematic survey of the Chrea National Park (Algeria) has made it possible to collect nineteen species of terrestrial Oligochaetes, which belong to the families of Lumbricidae, Megascolecidae and Acanthodrilidae. The Lumbricidae family dominates 94.39% and 16 species, the Species A. rosea, O. complanatus and A. caliginosa was found at all sites except Station Dhaia, often with very large populations; O. complanatus and A. rosea.
Acknowledgement
We are grateful to Mr R. Dahel, Director of Chréa National Park. We also thank the head of the El Hamdania sector and all his team for their help with the fieldwork. We also thank all members of the animal ecobiology laboratory for all their assistance to us, and their head is Mrs. Lebili Namcha, head of the laboratory.
References
Anderson, J.M., Ingram, J. (1993). Tropical soil biology and fertility programme: methods handbook. University of Exeter, UK.
Bachelier, G. (1963). La vie animale dans les sols. O.R.S.T.O.M. (Ed.), Paris, p:279.
Bachelier, G. (1972). Etude expérimentale de l’action des animaux sur l’humification des matériaux végétaux/I. Premières expériences et conclusions préliminaires. Coll Trav Dot. ORSTOM, Paris, p:175.
Baha, M. (1997). The earthworm fauna of Mitidja, Algeria. Tropical Zoology, 10:247-254.
Baha, M., Berra, S. (2001). Prosellodrilus doumandjii n. sp., a new lumbricid from Algeria. Tropical Zoology, 14:87-93.
Baha, M., Isserhane, W. (2017). Les oligochètes de dhaia (Parc National de Chréa). Revue ELWAHAT. University of Ghardaïa, Algérie.
Baldi, G., Texeira, M., Martin, O., Grau, H., Jobbagy, E. (2017). Opportunitiesdrive the global distribution of protected areas. Peer Journal, 5:e2989.
Bartz, M.L.C., Pasini, A., Brown, G.G. (2013). Earthworms as soil quality indicators in Brazilian no-tillage systems. Applied Soil Ecology, 69:39-48.
Beddard, F.E. (1992). On earthworms from Algeria and Tunisia. Proceedings of the Royal Physical Society of Edinburg, Session 1892, Edinburg.
Blanchart, E., Brauman, A. (2010). Les ingénieurs du sol: diversité et fonctions. Extrait du dossier thématique d’Agropolis International. Biodiversité. Des sciences pour les humains et la nature.
Bornebusch, C.H. (1930). The fauna of forest soil. Pet Forsttige Forsogsvasen, 11:1-158.
Bouché, M.B. (1972). Earthworms of France. Ecology and systematics. National Institute of Agronomic Research, Paris, pp:671.
Bouché, M.B. (2003). Vers de terre, de Darwin à nos jours Un révélateur heuristique. Académie des Sciences et lettres de Montpellier. Séance du. Montpellier, France.
Brown, G.G., Baroisa, I., Lavelle. P. (2000). Regulation of soil organic matter dynamics and microbial activityin the drilosphere and the role of interactionswith other edaphic functional domains. European Journal of Soil Biology, 36:177-198.
Brown, G.G., Pasini, A., Polo Benito, N., De Aquino, A.M., Correia, M.E.F. (2002). Diversity and functional role of soil macrofauna communities in brazilian no-tillage agroecosystems: A preliminary analysis. International Symposium on Managing Biodiversity in Agricultural Ecosystems, Montréal, Canada.
Brown, G.G., Domínguez, J. (2010). Use of earthworms as environmental bioindicators: principles and practices-the 3rd Latin American Meeting of Ecology and Taxonomy of Oligochaetes (ELAETAO3). Acta Zoological Mex, 26:2.
Bünemann, E.K., Bongiorno, G., Bai, Z., Creamer, R.E., De Deyn, G., De-Goede, R. (2018). Soil quality-a critical review. Soil Biology and Biochemistry, 120:105-125.
Capowiez, Y., Samartino, S., Cadoux, S., Bouchant, P., Richard, G., Boizard, H. (2012). Role of earthworms in regenerating soil structure after compaction in reduced tillage systems. Soil Biology and Biochemistry, 55:93-103.
Chapuis-Lardy, L., Le Bayon, R.C., Brossard, M., Lopez-Hernandez, D., Blanchart, E. (2011). Role of soil macrofauna in phosphorus cycling. In "Phosphorus in action-biological processes in soil phosphorus cycling", Bünemann, E.X., Oberson, A., Frossard, E. (Eds). Springer Soil Biology Series, Springer, NY, USA, pp:199-213.
Clarke, K.R., Warwick, R.M. (2001). Change in marine communities: an approach to statistical analysis and interpretation. 2nd Edition, PRIMER-E, Ltd., Plymouth Marine Laboratory, Plymouth.
Darwin, C. (1881). The formation of vegetable mould through the action of worms with observations on their habits. Murray, London.
Development Core Team. (2018). R: a language and environment for statistical computing. Version 3.4.4. Vienna: R Foundation for Statistical Computing.
Edward, C.A., Bohlen, P.J. (1996). Biology and ecology of earthworms. Chapman and Hall, London.
Edward, C.A. (2004). Earthworm ecology, 2nd ed, CRC Press, LLC, p:441.
El-Duweini, A.K., Ghabbour, S.I. (1965). Population density and biomass of earthworms in different types of Egyptian soils. Journal of Applied Ecology, 2:271-287.
Fiuza, D.T.F., Kusdra, J.F., Fiuza, S.D.S. (2012). Maize growth in soil with activity of giant earthworms Chibui bari (Oligochaeta: Glossoscolecidae). Brazilian Journal of Soil Science, 36:359-366.
Graff, O. (1962). Ein criodrilus aus südfrankreich. Vie et Milieu, 13:369-371.
Guggenberger, G., Haumaier, L., Thomas, R.J., Zech, W. (1996). Assessing the organic phosphorous status of an Oxisol under tropical pastures following native savanna using 31P NMR spectroscopy. Biology Fertilizer Soils, 23:332-339.
Hensen, V. (1877). Die Tätigkeit des Regenwurms (Lumbricus terrestris L.) für die Fruchtbarkeit des Erdbodens. Z. Wiss Zoology, 28:354-364.
Jimenez, J.J., Cepeda, A., Decaens, T., Oberson, A., Friesen, D.K. (2003). Phosphorus fractions and dynamics in surface earthworm casts under native and improved grasslands in a Colombian savanna oxisol. Soil Biology and Biochemistry, 35:715-727.
Johnston, A.S.A., Sibly, R.M., Thorbeck, P. (2017). Forecasting tillage and soil warming effects on earthworm populations. Journal of Applied Ecology, 55:1498-1509.
Kettler, T.A., Doran, J.W., Gilbert, T.L. (2001). Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Science Society of America Journal, 65:849-852.
Kinberg, J.G.H. (1867). Annulata nova. Ofvers K. Vetensk Akad. Förh. Stockh., 23:97-103.
Kuczak, C.N., Fernandes, E.C.M., Lehmann, J., Rondon, M.A., Luizao, F.J. (2006). Inorganic and organic phosphorus pools in earthworm casts (Glossoscolecidae) and a Brazilian rainforest Oxisol. Soil Biology and Biochemistry, 38:553-560.
Lamarck, J.B. (1800). Discours d’ouverture de l’an VIII, réédité dans. Bulletin Science Fr Belg, 40:459-482.
Lavelle, P. (1988). Assessing the abundance and role of invertebrate communities in tropical soils. Aims and methods. Journal of African Zoology, 102:275-283.
Lavelle, P., Spain, A.V. (1995). Soil ecology. Chapman and Hall, London.
Lavelle, P., Bignell, D., Lepage, M. (1997). Soil function in a changingworld: the role of invertebrate ecosystem engineers. European Journal of Soil Biology, 33:159-193.
Lavelle, P., Spain, A.V. (2001). Soil ecology. Kluwer Scientific Pubications, Amsterdam.
Lavelle, P., Decaënsb, T., Aubertb, M., Barota, S., Blouina, M., Bureaub, F., Margerieb, P., Moraa, P., Rossic, J.P. (2006). Soil invertebrates and ecosystem services. European Journal of Soil Biology, France.
Laverack, M.S. (1963). The physiology of earthworms. (Ed.) Pergamon Press, London, p:206.
Margalef, R. (1958). Temporal succession and spatial heterogeneity in phytoplankton. In: Buzzati-Treverso AA (ed.), Perspectives in Marine Biology, Berkeley, pp:323-349.
Meddour, R. (1994). Contribution to the phytosociological study of the central-eastern portion of Chréa National Park. Synthetic interpretation test of the stages and vegetation series of the Atlas Blidéen. Thesis Magister Science Agronomy, Institute of National Agronomy, Harrach, p:330.
Michaelsen, W. (1900). Oligochaeta. Das Tierreich, Friedländer (Ed.), Berlin, p:575.
Michaelsen, W. (1903). Die geographische Verbreitung der Oligochaeten. Friedländer and Sohn, Berlin. Bulletin of Zoological Nomenclature.
Michaelsen, W. (1930). Ein schlangenähnlicher regenwurm aus bergwäldern der insel luzon. Philippine Journal of Science, Manila, 41:273-282.
Misra, R. (1968). Ecology work Book. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, India.
Omodeo, P., Martinucci, G. (1987). Earthworms of Maghreb. In "On Earthworms", Bonvicini Pagliani, A.M., Omodeo, P. (eds.). Selected symposia and monographs, Mucci Modena, Italy, pp:235-250.
Omodeo, P., Rota, E., Baha, M. (2003). The megadrile fauna (Annelida: Oligochaeta) of Maghreb: a biogeographical and ecological characterization. Pedobiologia, 47:458-465.
Rosa, D. (1893). Revisione dei lumbricidi. Memorie dell'Accademia delle Scienze di Torino, 43:399-476.
Satchell, J.E. (1955). Some aspects of earthworm ecology. In: Soil Zoology (Ed.: D.K. Mc.E. Kevan). Butterworths, London, pp:180-201.
Savigny, J.C. (1826). Analyse des travaux de l’Académie Royale des Sciences pendant l’année 1821 partie physique. Member of Academic Royal Science Institute France, 5:176-184.
Sbabdji, M. (2012). Study of infestations in the Chréa cedar forest by the pine processionary, Thaumetopoea pityocampa Schiff spatiotemporal description and tree-defoliator relationship. Thesis Doctorate, Institute of National Agronomy, El Harrach.
Shannon, C.E., Weaver, W. (1963). The mathematical theory of communication, University of Illinois Press.
Sims, R.W., Gerard, B.M. (1999). Earthworms. FSC Publications, London, pp:167.
Tetry, A. (1937). Description d’une nouvelle espèce de Lombricien : Allolobophora cupulifera. Bulletin Society of Science Nancy, 10:119-123.
Vale, M.M., Tourinho, L., Lorini, M.L., Rajão, H. (2018). Endemic birds of the Atlantic Forest: traits, conservation status, and patterns of biodiversity. Journal of Field, Wiley Online Library.
Zajonc, I. (1959). Eisenia submontana (Vejd), un facteur important de la production forestière. Prir Časpid Slezský, 20:483-486.
Zeriri, I., Tadjine, A., Belhaouchet, N., Berrebbah, H., Reda-Djebar, M., Baha, M. (2013). Contribution to the identification of Oligochaeta: Lumbricidae in the region of Annaba in eastern Algeria. European Journal of Experimental Biology, 3:229-232.
Zhang, B.G., Li, G.T., Shen, T.S., Wang, J.K., Sun, Z. (2000). Changes in microbial biomass C, N and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biology and Biochemistry, 32:2055-2062.
Author Info
H. Zerrouki*, S. Hamil, M. Alili, W. Isserhane and M. BahaCitation: Zerrouki, H., Hamil, S., Alili, M., Isserhane, W., Baha, M. (2022). Contribution to the knoweldge of earthworm fauna of Chrea National Park (Algeria). Ukrainian Journal of Ecology. 12:1-10.
Received: 12-Feb-2022, Manuscript No. UJE-22-54309; Accepted: 07-Mar-2022, Pre QC No. P-54309; Editor assigned: 18-Feb-2022, Pre QC No. P-54309; Reviewed: 25-Feb-2022, QC No. Q-54309; Revised: 02-Mar-2022, Manuscript No. R-54309; Published: 15-Mar-2022, DOI: 10.15421/2022_348
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
