Research - (2022) Volume 12, Issue 4
Contribution for the study of zinc impact on a biological model “Lemna minor L”
A. Delimi1,2*, W. Boumaraf2, A. Bergal2, B. Dechir2 and S. Bouchelaghem1,3Abstract
Certain extensive used water treatment processes use duckweeds. Used water discharges are increasingly subject to different types of pollutions, particularly, heavy metals. In this work, we carry out an evaluation of a macrophyte species’ tolerance, the duckweed L. minor, to a heavy metal, zinc, through evaluating some indicative parameters for toxicity, such as the number of fronds, fresh weight and growth rate. Our results show that for a first time in a period of 21 days, the plant tolerates all the concentrations of the used zinc in our experiment from 0.003 to 0.018 g/l. Beyond that, it seems that L. minor reaches a threshold of reversible toxicity, demonstrated by the significant reduction in the fresh weight, fronds’ multiplication and growth rate, without exceeding 43% for the highest dose.
Keywords
L. minor, Zinc (Zn), Tolerance, Fresh weight, Fronds’, Accumulation.
Introduction
Environmental metal pollution has become one of the most important problems in the world. This phenomenon has increased in recent years due to the extensive use of heavy metals in agriculture, chemistry and industries. Heavy metals often have harmful influences on the health of living beings, especially humans. Direct and/or indirect harmful effects may occur from the molecular level to communities and ecosystems (Grara etal., 2012).
Phytoremediation is defined as the use of plants for the reduction and/or elimination of pollutants in soil and water, more particularly heavy metals. Phytoremediation is carried out naturally by plants surviving in water (rhizofiltration, phytostabilization or phytofiltration) and with contaminated soils (phytoextraction, phytodegradation, phytovolatilization, and rhizo-degradation) (Dushenkov et al., 1995; Faisal et al.,2004).
Polluted waters, which can be treated by phytoremediation, include, used waters and municipal used waters (nutrients, metals), agricultural runoff/drainage water (fertilizers, nutrients, metals, arsenic, selenium, boron, pesticides organic), industrial used waters (metals, selenium), lixiviats discharge, drainage waters (metals) and groundwater (organic, metals) (Raskin et al., 1997; Ferro et al., 2001).
Among the purifying plants, duckweeds constitute a good experimental model given their fast growth, the ease of culture and harvesting. Certainly, they have been widely used to evaluate the pollutants’toxicity, such as: industrial oils (Tkalec etal., 1998), heavy metals (Mohan and Hosetti, 1997; Wang, 1986), hydrocarbons (Huang et al., 1995) and pesticides (Hartman and Martin, 1985).
In duckweeds, the effect of toxic substances can be estimated by different parameters such as the number of fronds, biomass and leaf surface (Tkalec et al., 1998; Wang, 1986). The number of fronds (NF) and biomass (FW), in addition to their wide use, present the advantage of being simple and compatible with a large experimental setup. It is in this perspective that the objective of this work is to evaluate the zinc tolerance (Zn) in L. minor.
Materials and Methods
Our biological model L. minor, used in this study is collected during a field trip to Tonga Lake in El Kala’s National Park, which is located in the extreme north-east of Algeria. The harvested fronds are washed several times with distilled water before being seeded in trays, containing a solution of water and zinc (Zn). 100 fronds of each species were seeded in plastic pots, the pH was adjusted to 6.8. In addition to the control ones, treatments corresponding to the following (Zn) concentration gradients were used: 0-0.003-0.005-0.01-0.012-0.015-0.018 g. each treatment was repeated three times. Water losses by evapotranspiration, we add 15 mL of the nutrient solution daily. A daily estimate of our samples’ fresh weight was launched on the first day of the experiment, for six days. A follow-up of fresh weight was then carried out, every weekend for a period of a month. The monitoring of growth kinetics through counting the number of fronds NF and fresh weight gain (FW), was carried out for a month at the rate of once per weekend. The growth rate (DNF) is estimated by the difference between the number of the initial and final fronds (Wang, 1986).
Results and Discussion
Fresh weight of L. minor growth in (Zn)
The obtained results for the daily intake of fresh weight in six days show a consistent increase in (Fw) for all concentrations during the first six days. Certainly, it seems that during the 1st week Zinc does not have a toxic or growth inhibitor effect on L. minor, since the development is regular for all the (Zn) concentrations of our samples (Fig. 1). However, our trial continues over time by taking (FW) for a month, since taking it every weekend shows an increase in the latter for all concentrations until the third week. On the other hand, we observed a decrease in (FW) during the 4th week for the last four (Zn) concentrations from 0.01 g/l to 0.018 g/l (Fig. 2). According to (Hutchinson and Czyrska, 1975), the period of treatment is also an important parameter in this type of experiment which can influence the measurement of the plants’ sensitivity to metals. Therefore, in Lemna valdiviana, a one week treatment with Cd generates 50% of growth inhibition at 0.2 mg Cd/L, but, with two weeks’ treatment, the same result is obtained at only 0.03 mg Cd/I.
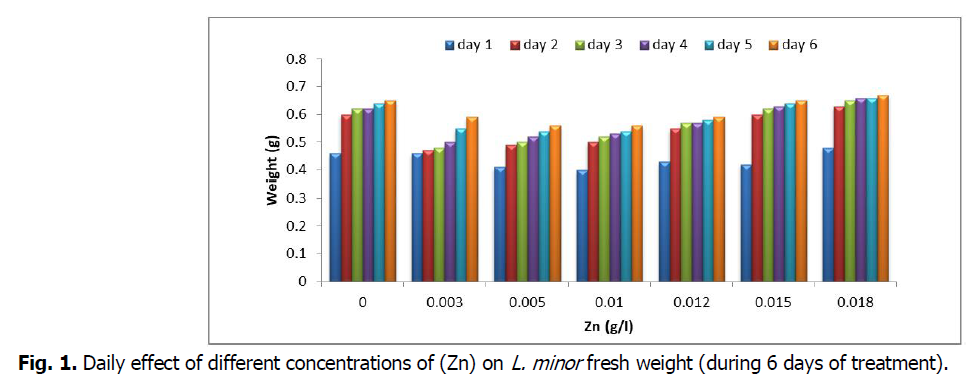
Fig 1. Daily effect of different concentrations of (Zn) on L. minor fresh weight (during 6 days of treatment).

Fig 2. Weekly effect of different (Zn) concentrations on L. minor fresh weight.
In fact, (Megateli et al.,2014), reported that L. gibba showed a good efficiency, in the elimination of zinc, cadmium and copper, where the elimination rates of 100, 90 and 77% were separately recorded after 10 days of treatment. These performances are superior to those obtained in the presence of Myriophylhum aquaticum, Ludwigina palustris and Mentha aquatica (42 and 34% of copper and zinc’s elimination, respectively) after two weeks of culture (Kamal et al., 2004). According to Chafaa, et al., (2015), Lemna minor seemed more suitable for biomonitoring and phytoremediation applications than Spyrogyralink sp, Fontinalis antipyretica, taking into account its tolerance and biomass productivity.
This may explain the increase in the biomass (FW) of our samples over a period of time, since L. minor has the ability to initially eliminate zinc. However, the extension of the treatment period by one month induces a decrease in (FW), particularly for the highest doses of (Zn) from 0.01g/l. This decrease in the biomass makes it possible to deduce that L. minor, is no longer able to eliminate Zinc in a second phase.
Number of fronds of L. minor growth in (Zn)
Concerning the number of fronds parameter (Fig. 3), we have also noticed a multiplication of fronds (NF) on the different concentrations for the first three weeks, and a decrease in the number of fronds during the fourth week. This is observed beyond concentration (0.01 g/l).

Fig 3. Weekly effect of different concentrations of (Zn) on the number of L. minor fronds.
According to Ater et al., (2006), the effect of (Cu) on NF is such that, for L. minor, all the concentrations tested are phytotoxic. In the absence of (Cu), the Number of fronds (NF) is approximately 115 fronds, whereas in the presence of 1 mg Cu/L, the number drops to less than 80 fronds.
A similar study on tolerance and accumulation of copper and chromium in two species of duckweed: L. minor L. and L. gibba L, shows that both species are sensitive to copper. In fact, they observed a highly significant inhibition of the expressed growth both by the multiplication of fronds (NF) and the biomass or the fresh weight, in response to the concentration gradient used (Ater et al., 2006).
Rate of growth (DNF)
Concerning the rate parameter of L. minor growth, after three weeks of treatment (21 days), we noted a significant decrease in the rate of DNF with an increase in (Zn) doses (Fig. 4). It goes from 88% with the first dose of (Zn) 0.003 g/l to 43% with the highest dose of (Zn) 0.018 g/l. In fact, the rate parameter of growth indicates that L. minor is sensitive to gardions of concentration in (Zn) after a period. The obtained results show a significant and dose-dependent decrease in the growth rate, this decrease is of the order of 60% compared to the control one.

Fig 4. Effect of different concentrations (Zn) on L. minor growth rate during a month of treatment.
It also seems that L. minor is much more sensitive than L. gibba. The growth in L. minor is more significantly affected. The toxicological parameters reflect this observation: L. minor has both a CSEO and an IC50 lower than those of L. gibba. (Ater et al.,2006).
However, it should be noted that the comparisons with the bibliographic data must be put into perspective, since the sensibility measurement to metals can be influenced by several factors, directly related to the nature of the used experimental devices. For example, it has been shown in another species of duckweed, Lemna paucicostata, that pH, nutrient solution composition, and temperature can affect the sensibility (Nasu and Kugimoto, 1981).
Different studies have shown a high accumulation of trace elements in L. minor (Mo et al.,1989; Zayed et al., 1998). According to Mallick et al., (1996), L. minor is more efficient for (Zn) and Cr accumulation than Azollapinnata and Lemna gibba. Also, in other several moss species (Ah-peng, 2003), algae (Kaimoussi et al., 2004), and duckweed (Ater et al., 2006), the order of metals’ importance is: (Zn)>Cu>Pb>Hg. For the same concentration in the environment, (Zn) accumulates faster than other heavy metals such as (Cu) and (Pb). The decreasing ranking of metals found in their study has already been shown by various authors and for different species (Chafaa et al., 2015).
By the end of this study, we can conclude that L. minor constitutes a good experimental model given their fast growth, the ease of cultivation and harvesting. In fact, they have been widely used for the evaluation of pollutants toxicity, such as industrial oils, heavy metals, hydrocarbons and pesticides. (Ater et al., 2006).
Conclusion
The results of the experiments related to the study of zinc effects on a biological model Lemna minor, make it possible to draw a certain number of conclusions:
L. minor has a very interesting (Zn) accumulation capacity during a first time, more than 21 days, resulting in weight gain and an increase in the number of fronds for all of (Zn) concentrations.
The extension of the experiment’s period shows that Zinc becomes toxic, because it provokes irreversible disturbances in L. minor at a certain zinc concentration. This causes a drop in the biomass and the number of founds, with a decrease up to 43% in the growth rate for the highest concentration of 0.018 g/l.
L. minor, presents a good biological model for the problems’ detection due to micro pollutants and heavy metals, through the use of the studied growth parameters such as: The fresh weight, the number of fronds and the growth rate. The plant is also a good experimental model for phytoremediation research given the fast growth, ease of cultivation and harvesting.
Given its tolerance, accumulation capacity and biomass productivity, Lemna minor can be exploited in phytoremediation applications in our country.
References
Ah-Peng, C. (2003). Mise au point d’un outil diagnostic basé sur l’utilisation de la mousse aquatique Fontinalis antipyretica Hedw en culture pour l’estimation de la qualité des cours d’eau. Thèse de doctorat, Université de Lille II, Lille, p:187.
Ater, M., Aït Ali, N., Kasmi, H. (2006). Tolérance et accumulation du cuivre et du chrome chez deux espèces de lentilles d’eau: L. minor L. et L. gibba L. Revue des sciences de l'eau/Journal of Water Science, 19:57-67.
Chafaa, M., Maatoug, M., Tandlich, R., Benchaben, H., Ait Hammou, M. (2005). Bio-surveillance des metaux lourds (pb, (Zn), cu) a la sortie de la station d’epuration de Tiaret (Algerie) au moyen des vegetaux aquatiques: plante Lemna Minor, Algue Spyrogyra Link Sp Et Broyophyte Fontinalis Antipyretica. European Scientific Journal, 11:155-174.
Dushenkov, V.P., Nanda Kumar, B.A., Motto, H. (1995). Rhizofiltration: The use of plants to remove heavymetals from aqueous streams”. Environmental Science and Technology, 29:1239-1245.
Faisal, I., Khan, T.H., Ramzi, H. (2004). An overview and analysis of site remediation technologies”. Environmental Management, 71:95-122.
Ferro, A., Chard, J., Kjeldren, R., Turner, D., Montague, T. (2001). Ground water capture usinghybridpoplartrees: evaluation of a system in Ogden, Utah. International Journal of Phytoremedy, 3:87-104.
Grara, N., Ataillia, A., Boucenna, M., Berrebbah, H., Djebar, M.R. (2012). Toxicity of metal dust from Annaba steel complex (Eastern Algeria) on the morpho-physiological parameter of the snail Helix aspersa. Advanced in Biology and Environnement, pp:605-611.
Hartman, W.A., Martin, D.B. (1985). Effect of four agricultural pesticides on Daphniapulex, Lemna minor and Potamogetonpectinatus. Bulletin Environment Contamination Toxicity, 35:646-651.
Huang, X.D., Dixon, D.G., Greenberg, B.M. (1995). Increased polycyclic aromatic hydrocarbon toxicity following their photomodification in natural sunlight. Ecotoxicity Environmental Safety, 32:194-200.
Hutchinson, T.C., Czyrska, H. (1975). Heavy metals toxicity and synergism to floating aquaticweeds. Verh International Verein Limnology, 19:2101-2111.
Kaimoussi, A., Mouzdahir, A., Abdelkbir, S. (2004). Variations saisonnières des teneurs en métaux (Cd, Cu, Fe, Mn et (Zn)) chez l’algue Ulva lactuca prélevée au niveau du littoral de la ville d’El Jadida (Maroc). Plant Biology and Pathology, 327:361-369.
Kamal, M., Ghaly, A.E., Mahmoud, N., Côté, R. (2004). Phytoaccumulation of heavymetals by aquatic plants. Environmental International. 29:1029-1039.
Mallick, N., Sharden, D.U., Rail, C. (1996). Removal of heavy metals by two free floating aquatic macrophytes. Biomedical Environmental Science, 9:399-407.
Megateli, S., Brahim, N., Couderchef, M. (2014). La phytoremédiation: avantages et limites d'applications. Revue Agrobiologia, 6:42-46.
Mo, S.C., Choi, D.S., Robinson, J.W. (1989). Uptake of mercury from aqueous solution by duckweed, the effects of pH, copper and humic acid. Journal of Environmental Science and Health, 24:135-146.
Mohan, B.S., Hosetti, B.B. (1997). Potentialphy to toxicity of lead and cadmium to Lemna minor grown in sewage stabilisation ponds. Environmental Pollution, 2:233-238.
Nasu, Y., Kugimoto, M. (1981). Lemna (Duckweed) as an indicator of water pollution. I The sensivity of Lemnapaucicostata to heavymetals. Architech Environmental Contamination Toxicity, 10:159-169.
Raskin, I., Smith, R.D., Salt, D.E. (1997). Phytoremediation of metals: using plants to remove pollutants from the environment. Current Opinion Biotechnology, 8:221-226.
Tkalec, M., Vidakovic, C.Z., Regula, I. (1998). The effect of oil industry «high density brines» on duckweed Lemna minor L. Chemosphere, 13:2703-2715.
Wang, W. (1986). Toxicity tests of aquatic pollutants by using common Duckweed. Environmental Pollution, 11:1-14.
Zayed, A., Gowthaman, S., Terry, N. (1998). Phytoaccumulation of trace elements by wetland plants: I. Duckweed. Journal of Environmental Quality, 27:715-721.
Author Info
A. Delimi1,2*, W. Boumaraf2, A. Bergal2, B. Dechir2 and S. Bouchelaghem1,32Department of Biology, Faculty of Natural and Life Sciences, University of Chadli Bendjedid El Tarf, Algeria
3Department of Agronomy. Faculty of Natural and Life Sciences, University of Chadli Bendjedid El Tarf, Algeria
Citation: Delimi, A., Boumaraf, W., Bergal, A., Dechir, B., Bouchelaghem, S. (2022). Contribution for the study of zinc impact on a biological model “Lemna minor L”. Ukrainian Journal of Ecology. 12:68-72.
Received: 11-Apr-2022, Manuscript No. UJE-22-60225; , Pre QC No. P-60225; Editor assigned: 13-Apr-2022, Pre QC No. P-60225; Reviewed: 27-Apr-2022, QC No. Q-60225; Revised: 02-May-2022, Manuscript No. R-60225; Published: 07-May-2022, DOI: 10.15421/2022_366
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
