Research - (2021) Volume 0, Issue 0
Comprehensive evaluation of spring barley yield and tolerance to abiotic and biotic stresses
V.M. Hudzenko1*, O.A. Demydov1, T.P. Polishchuk1, I.V. Fedorenko1, A.A. Lysenko1, M.V. Fedorenko1, A.A. Siroshtan1, T.V. Yurchenko1 and T.V. Shevchenko2Abstract
The results of comprehensive evaluation in 2018-2020 of 96 spring barley accessions of different ecological origin under conditions of the central part of the Ukrainian Forest-Steppe are presented. As a result of field trials and laboratory investigation, the new genetic sources in terms of high and stable grain yield, relative tolerance to drought, lodging resistance, and resistance to diseases have been identified. Accordingly to the GYT (genotype by yield*trait) biplot, the accessions Skald (POL), Despina (DEU), Kormoran (POL), Almonte (CAN), Suveren (POL), Dar Nosivshchyny (UKR), Scarb (POL), Vienna (AUT), AC Alma (CAN), Smarahd (UKR) are characterized with increased yield and combination of a set of adaptive traits. The new genetic sources identified are valuable in spring barley breeding to develop an initial material with combination of yield, its stability, and tolerance to the most widespread abiotic and biotic stresses under conditions of the central part of Ukrainian Forest-Steppe.
Keywords
Barley, disease, drought, GYT biplot, yield, lodging, stress.
Introduction
Barley (Hordeum vulgare L.) is one of the main cereal crops worldwide. Ukraine is one of the largest producers and exporters of barley grain (FAO, 2021). A significant increase in barley yield since the middle of the twentieth century is directly related to the development and releasing of new varieties (Lillemo et al., 2009; Laidig et al., 2017). The effectiveness of plant breeding, in turn, is largely dependent on the availability of a sufficient amount of genetically diverse source material (Darrier et al., 2019; Milner et al., 2019). That is why the formation, preservation and maintenance of viable genetic collections and their systematic study are determined as priorities for food security of mankind (Hopkin, 2008; Statkevičiūtė and Leistrumaitė, 2010; Govindaraj et al., 2015). The narrowing of genetic diversity due to the modernization of agricultural production has been called genetic erosion (van de Wouw et al., 2010; Megersa, 2014; Gadissa et al., 2021). The most typical is the reduction of genetic diversity due to the replacement of local forms (e.g., landraces) with commercial varieties from the elite gene pool. To investigate barley genetic diversity, various molecular genetic methods have been implemented at an unprecedented rate (Pasam et al., 2014; Ferreira et al., 2016; Sato, 2020). The results of studies on the presence or absence of signs of narrowing the genetic basis of modern varieties differ (Russell et al., 2011; Zhou et al., 2012; Ovesná et al., 2013). In European varieties of different breeding periods, it was found that genetic diversity was not stable during the last century. Both loss and involve of new alleles are noted. Therefore, when characterizing the genetic diversity of barley in Europe, it should be considered as a dynamic system that is constantly changing without an obvious one-way (Malysheva-Otto et al., 2007). That is, modern plant breeding does not necessarily lead to narrowing the genetic basis. On the contrary, in breeding programs which constantly involved a different source material, the widening genetic diversity of varieties and increasing the potential of their productivity have been observed (Koebner et al., 2003; Poets et al., 2016). Thus, the evaluation and participation of new genetic diversity in the breeding process has never lost its relevance, and due to the intensification of modern breeding and global climate change, it has become increasingly important (Dawson et al., 2015; Panfilova et al., 2020). It should be noted that despite the development of molecular and genetic research, the assessment of collection accessions on valuable agronomic traits does not lose its value in practical breeding (Brantestam et al., 2014; Jairus et al., 2015; Dinsa et al., 2018; Sayd et al., 2019). In addition, it is known that the same accessions may have different breeding value in different ecological niches due to different weather conditions, soil types, air and water regimes, photoperiodical mode, different races of pathogens, and many other factors (Comadran et al., 2011; Lillemo et al., 2019). Therefore, it is necessary to experimentally verify the value of genetic sources under the conditions where breeding is carried out. Our previous long-term research has shown that the most characteristic manifestation of adverse weather conditions in the environment of the the central part of Ukrainian Forest-Steppe were increased air temperatures, their sharp amplitude of fluctuations, and strong uneven precipitation during the spring barley growing season (Gudzenko and Vasylkivsky, 2016). In some years, this can lead to different combinations of a number of adverse phenomena that may significantly reduce spring barley yield. They are the deficit of available moisture, temperature stress, lodging due to heavy rains, and intensive spreading of pathogens (powdery mildew, leaf spots, and leaf rust) (Hudzenko et al., 2017). Therefore, under conditions in the central part of the Ukrainian Forest-Steppe, the need to develop spring barley varieties with a combination of high yield potential, genetically determined tolerance to high air temperature and moisture deficit in the different stages of vegetation, resistance to lodging, as well as resistance to the complex of the most widespread diseases has been determined. Based on the above, the aim of the study was to reveal the peculiarities in the variation in the yield variation of spring barley collection accessions of different ecological origin in weather-contrasting years under conditions of the central part of the Ukrainian Forest-Steppe, as well as to identify the new genetic sources of individual or combination of agronomic and adaptive traits.
Materials and Methods
The study was conducted at the V.M. Remeslo Myronivka Institute of Wheat of National Academy of Agrarian Sciences of Ukraine (MIW) in 2018-2020. Geographical coordinates are: latitude-49°64', longitude-31°08', altitude-153 m. The soils are deep, with little humus and slightly leached chornozem. Humus content 3.8%, alkaline hydrolyzed nitrogen 59 mg kg-1, P2O5-221 mg kg-1, K2O-96 mg kg-1, pH=5.8. The subjects of the study were 96 spring barley collection accessions originating from 15 countries (Table 1).
| Code | Accession | Botanical Variety | Origin | Code | Accession | Botanical Variety | Origin |
|---|---|---|---|---|---|---|---|
| G1 | Vzirets (standard) | nutans | UKR | G50 | Antigone | nutans | GBR |
| G2 | Novator | inerme | UKR | G51 | Stalyi | nutans | UKR |
| G3 | Revansh | inerme | UKR | G52 | Baskak | nutans | UKR |
| G4 | Phoenix | nudum | CAN | G53 | Tiver | nutans | UKR |
| G5 | CDC Cartel | nudum | CAN | G54 | Avers | nutans | UKR |
| G6 | 4-15 | nudum | UKR | G55 | JaK 401 | deficiens | RUS |
| G7 | 4-14 | nudum | UKR | G56 | Aristei | deficiens | UKR |
| G8 | 4-9 | nudum | UKR | G57 | Shchedryk | deficiens | UKR |
| G9 | 4-2 | nudum | UKR | G58 | Pamjati Raisy | medicum | KAZ |
| G10 | 4-1 | nudum | UKR | G59 | Karagandinskij 5 | medicum | KAZ |
| G11 | Nudum 95 | nudum | RUS | G60 | Karagandinskij 7 | submed | KAZ |
| G12 | Rosalina | nudum | DNK | G61 | Tuleevskij | pallidum | RUS |
| G13 | CDC Candle | nudum | CAN | G62 | Omskij 99 | pallidum | RUS |
| G14 | CDC Alamo | nudum | CAN | G63 | Brier | pallidum | USA |
| G15 | NSGJ-1 | nudum | SRB | G64 | Yerong | pallidum | AUS |
| G16 | AC Alberte | nudum | CAN | G65 | Nord | pallidum | CAN |
| G17 | L 94 | nigrinud | DEU | G66 | AC Westech | pallidum | CAN |
| G18 | Jet | nigrinud | CAN | G67 | AC Maple | pallidum | CAN |
| G19 | Karat | nutans | RUS | G68 | AC Alma | pallidum | CAN |
| G20 | Severjanin | nutans | RUS | G69 | Kaz'minskij | ricotense | RUS |
| G21 | Batik | nutans | RUS | G70 | Nobarb | ricotense | CAN |
| G22 | Zubr | nutans | BLR | G71 | AC Vision | ricotense | CAN |
| G23 | Arat | nutans | RUS | G72 | Glacier AL.38 | pallidum | GBR |
| G24 | Orenburgskij sovmestnyj | nutans | RUS | G73 | Millhouse | nudum | CAN |
| G25 | Medikum 139 | nutans | RUS | G74 | Dar Nosivshchyny | nutans | UKR |
| G26 | Kredo | nutans | RUS | G75 | Smarahd | nutans | UKR |
| G27 | Stepan | nutans | RUS | G76 | Sviatovit | nutans | UKR |
| G28 | Cheljabinec 2 | nutans | RUS | G77 | Reindzher | nutans | UKR |
| G29 | Kuralaj | nutans | KAZ | G78 | Bukat | nutans | UKR |
| G30 | Syr-aruy | nutans | KAZ | G79 | Fest | nutans | BLR |
| G31 | Symbat | nutans | KAZ | G80 | Strief | nutans | DEU |
| G32 | Vatan | nutans | KGZ | G81 | Skarb | nutans | POL |
| G33 | Shynar | nutans | KGZ | G82 | Sunshine | nutans | DEU |
| G34 | Zolotnik | nutans | RUS | G83 | Concerto | nutans | GBR |
| G35 | Ergeninskij 2 | nutans | RUS | G84 | Henrike | nutans | DEU |
| G36 | Muson | nutans | RUS | G85 | Almonte | nutans | CAN |
| G37 | Natali | nutans | RUS | G86 | Diplom | nutans | DEU |
| G38 | Jastreb | nutans | RUS | G87 | Victoriana | nutans | DEU |
| G39 | Abalak | nutans | RUS | G88 | Mastvinster | nutans | DEU |
| G40 | Bahus | nutans | RUS | G89 | Jermina | nutans | GBR |
| G41 | Olenjok | nutans | RUS | G90 | Biatlon | nutans | GBR |
| G42 | Radzіmіch | nutans | BLR | G91 | Kaputar | nutans | AUS |
| G43 | Vladlen | nutans | KGZ | G92 | Vienna | deficiens | AUT |
| G44 | Azyk | nutans | KAZ | G93 | Kormoran | deficiens | POL |
| G45 | Karagandinskij 6 | nutans | KAZ | G94 | Skald | deficiens | POL |
| G46 | Ilek 16 | nutans | KAZ | G95 | Suveren | deficiens | POL |
| G47 | KAZSUFFLE 1 | nutans | KAZ | G96 | Despina | deficiens | DEU |
| G48 | Chugaki No. 14 | nutans | MNG | G97 | Lilly | deficiens | DEU |
| G49 | Krok | nutans | UKR | - | - | - | - |
Table 1. List of barley accessions involved in the study along with their origin and botanical variety, 2018-2020.
Genetic diversity is represented by nine botanical varieties that belong to two groups (covered and naked) and two subspecies (six-rowed and two-rowed). The trial was carried out with a complete randomized complete block design in three replications. The net plot size was 1 square meter. The spring barley variety Vzirets was used as a standard, which was planted every 20 plots.
The assessment of resistance to diseases was performed under natural field conditions according to the methodical recommendation (Babayants et al., 1988). Evaluation of relative drought tolerance was determined with the barley seed germination method of barley seeds germination in a sucrose solution with an osmotic pressure of 12 atmospheres (Dorofeev et al., 1974). The accessions were divided into five groups according to the level of relative tolerance to drought. Group I (high tolerance) germinated more than 81% of seeds in sucrose solution compared to control (distilled water), group II (above average tolerance) germinated 61-80% of seeds, group III (average tolerance) germinated 41-60% seeds, IV (low tolerance)-21-40% of seeds, group V (very low tolerance) germinated 0-20% of seeds. The experimental data were processed with the Microsoft Excel 2010 and Statistica 12.
For comprehensive evaluation accessions in terms of combination of increased yield with tolerance to abiotic and biotic stresses, a GYT (genotype by yield*trait) biplot was applied. GEA-R software was used to construct graphical visualizations (Pacheco et al., 2015).
Results and Discussion
Weather conditions of the pre-sowing period and during the growing season differed depending on years of research, and also varied relative to the average long-term data. Fluctuations in the hydrothermal regime clearly characterize the coefficient of significance of deviations of actual meteorological data from the long-term average. For the average monthly air temperature, this indicator points to a general tendency towards its increasing (Fig. 1).

Fig 1. Coefficient of significance of the deviation of actual average monthly air temperature from the long-term average in pre-sowing and during the growth periods of spring barley.
Especially critical in terms of high air temperature in 2018 were April and May, to a lesser extent June, in 2019 was June, and less were March and May, in 2020 were March and June. During the study period, as an exception, significant deviations of the average monthly air temperature in the direction of its decrease were observed only in March 2018 and May 2020. At the same time, the amount of monthly precipitation can be observed almost directly opposite (Fig. 2).
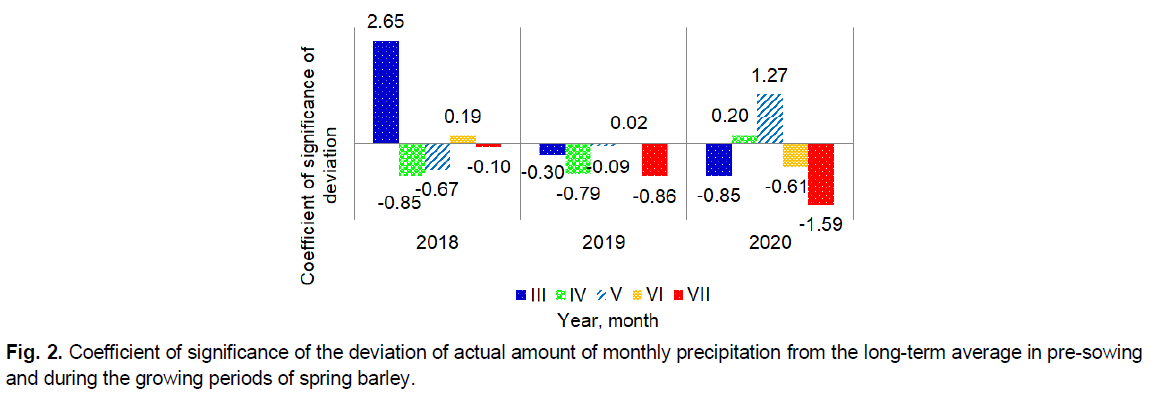
Fig 2. Coefficient of significance of the deviation of actual amount of monthly precipitation from the long-term average in pre-sowing and during the growing periods of spring barley.
The general trend indicates a decrease in precipitation in most months over the years of research. A significant predominance of precipitation on a long-term average was observed only in March 2018 and May 2020. That is, in those months when there was a significant decrease in temperature.
Drought is a complex abiotic stress that can have varying degrees, duration, and effects at different periods of barley plant development (Arshadi et al., 2018; Jabbari et al., 2018; Zargar et al., 2018; Filip et al., 2020). Due to climate change and the increasing frequency of early spring drought cases in the central part of Ukrainian Forest-Steppe, the ability of the spring barley primary root system to use winter moisture reserves quickly and efficiently is of great importance. The ability of seeds to germinate under artificial physiological drought is related to their potential ability to germinate in the absence of moisture under natural conditions. It was noted that this capacity was related to the formation of a stronger primary root system, which makes a significant contribution to the further tolerance to drought tolerance of the adult plant (Wehner et al., 2016; Thabet et al., 2018; Abdel-Ghani et al., 2019). In our study, on average in 2018-2020, the majority of accessions had very low or low tolerance (Fig. 3). There were only five accessions, G5 CDC Cartel (CAN), G16 AC Alberte (CAN), G13 CDC Candle (CAN), G4 Phoenix (CAN), and G6 4-15 (UKR), which were characterized with high relative drought tolerance. Six accessions G10 4-1 (UKR), G9 4-2 (UKR), G7 4-14 (UKR), G11 Nudum 95 (RUS), G73 Millhouse (CAN) and G66 AC Westech (CAN) had above average tolerance. Also, six accessions G 62 Omskii 99 (RUS), G61 Tuleevskij (RUS), G14 CDC Alamo (CAN), G67 AC Maple (CAN), G17 L94 (DEU) and G12 Rosalina (DNK) had average relative drought tolerance.

Fig 3. Distribution of accessions according to the level of relative drought tolerance, on average 2018-2020.
In the context of climate change, the resistance of barley to lodging does not only does not lose relevance, but, conversely, requires systematic research (Dockter and Hansson, 2015; Mikoajczak et al., 2017). The highest degree of lodging in accessions was observed in 2018, and the lowest was in 2019. On average for three years, only eight accessions G83 Concerto (GBR), G90 Biatlon (GBR), G67 AC Maple (CAN), G80 Strief (DEU), G84 Henrike (DEU), G94 Skald (POL), G79 Fest (BLR), G91 Kaputar (AUS) had very high resistance to lodging (9 points) (Fig. 4).
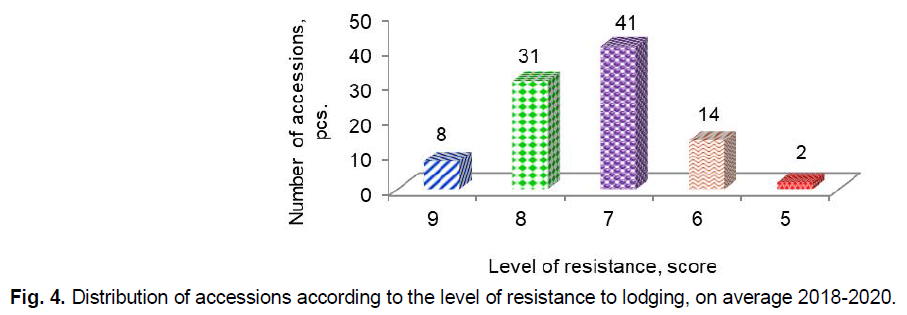
Fig 4. Distribution of accessions according to the level of resistance to lodging, on average 2018-2020.
Powdery mildew caused by Blumeria graminis (DC.) E.O. Speer, F. sp. hordei emend. É.J. Marchal (anamorph Oidium monilioides Link) is a foliar disease of barley of worldwide importance that can leads to yield losses of up to 30% (Bengtsson et al., 2017; Hoseinzadeh et al., 2019; Pogoda et al., 2020; Dreiseitl, 2020). The largest development of powdery mildew was observed in 2019. On average in 2018-2020 only 12 accessions G50 Antigone (GBR), G83 Concerto (GBR), G90 Biatlon (GBR), G80 Strief (DEU), G17 L 94 (DEU), G88 Mastvinster (DEU), G96 Despina (DEU), G93 Kormoran (POL), G12 Rosalina (DNK), G5 CDC Cartel (CAN), G7 4-14 (UKR), G56 Aristei (UKR) had high resistance (8 points) (Fig. 5). Two other very damaging diseases of barley are net blotch caused by Pyrenophora teres Drechsler (anamorph Drechslera teres [Sacc.] Shoem.), and spot blotch caused by Cochliobolus sativus (anamorph Bipolaris sorokiniana [Sacc.] Shoem.).
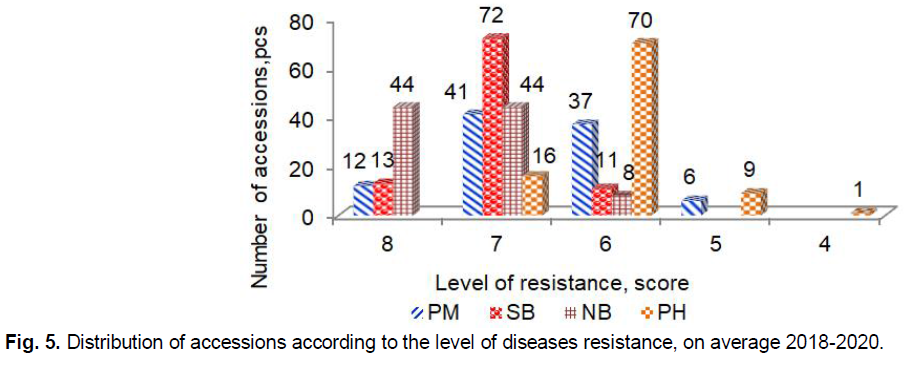
Fig 5. Distribution of accessions according to the level of diseases resistance, on average 2018-2020.
Both diseases can lead to yield losses of up to 40%, with the potential for total plants to fail if susceptible cultivars are planted under favorable environmental conditions for pathogens environmental conditions (Clare et al., 2020; Visioni et al., 2020). In our study, the highest manifestation of net blotchwas observed in 2018 and spot blotch was in 2019. Resistance to net blotch (8 points) showed 44 accessions, including G3 Revansh (UKR), G54 Avers (UKR), G39 Abalak (KAZ), G27 Stepan (RUS), G26 Kredo (RUS), G18 Jet (CAN), G66 AC Westech (CAN), G5 CDC Cartel (CAN), G65 Nord (CAN), G15 NSGJ-1 (SRB), G17 L 94, (DEU), G31 Symbat (KAZ), G29 Kuralaj (KAZ) G46 Ilek 16 (KAZ), and others. High resistance (8 points) to spot blotch had only 13 accessions G68 AC Alma (CAN), G66 AC Westech (CAN), G18 Jet (CAN), G71 AC Vision (CAN), G62 Omskij 99 (RUS), G27 Stepan (RUS), G55 JaK 401 (RUS), G21 Batik (RUS), G34 Zolotnik (RUS), G24 Orenburgskij sovmestnyj (RUS), G26 Kredo (RUS) G28 Cheljabinec 2 (RUS), and G77 Radzimich (BLR). The biotrophic fungus Puccinia hordei Otth. the causal agent of barley leaf rust is also an important pathogen in many barley growing areas worldwide that causes yield losses in general about 15-25% and, under favorable conditions, cause losses of up to 60% (Vatter et al., 2018; Fazlikhani et al., 2019). Leaf rust developed strongly in 2018 and 2019. We did not identify accessions with immunity (9 points) or high resistance (8 points) to Puccinia hordei. Resistant (7 points) to leaf rust were 16 accessions G95 Suveren (POL), G97 Lilly (DEU), G96 Despina (DEU), G87 Victoriana (DEU), G88 Mastvinster (DEU), G86 Diplom (DEU), G89 Jermina (GBR), G83 Concerto (GBR), G50 Antigone (GBR), G90 Biathlon (GBR), G34 Zolotnik (RUS), G38 Jastreb (RUS), G5 CDC Cartel (CAN), G73 Millhouse (CAN) G4 Phoenix (CAN), and G74 Dar Nosivshchyny (UKR).
The highest values in breeding programs have accessions that are characterized not only by high manifestation of individual traits but their combination, and especially with increased yield performance. For genotype selection based on yield and trait complex combination, a novel GYT biplot approach was proposed (Yan and Frégeau-Reid, 2018). As mentioned by the authors, the background for the GYT biplot is a paradigm shift, according to which genotypes should not be evaluated not on discrete traits, but on the basis of yield combination with a set of other important agronomic important traits. The GYT biplot makes it possible to characterize genotypes on the basis of such a combination of yield with other parameters (these may be elements of yield structure, grain quality parameters, tolerance to abiotic and biotic factors, etc.) and simultaneously identify their strengths and weaknesses.
In the first stage of GYT biplot analysis, the experimental data were modified by multiplying them with yield. For a comprehensive evaluation of accessions, we included all the traits characterized traits. As a result, the following yield*trait combinations were obtained: yield and relative drought tolerance (YLD_DT), yield and resistance to lodging (YLD_LD), yield and resistance to powdery mildew (YLD_PM), yield and resistance to net blotch (YLD_NB), yield and resistance to spot blotch (YLD_SB), and yield and resistance to leaf rust (YLD_PH). Based on the intermediate GYT table (not given), standardized index values were obtained. To do this, the results of the yield*trait combination of a particular genotype were subtracted from the mean value in the trial, followed by a division on standard deviation. On the basis of a number of modified yield*trait combinations, the general (mean) GYT index was determined, which characterizes the complex value of each genotype (not given). Based on the data obtained, a graphical analysis was performed (Fig. 6). The principles of GYT biplot graph construction are the same as for the already well-known GGE biplot (Yan and Tinker, 2006). The difference is that the term ‘environment’ is replaced by a combination of ‘yield*trait’. The first two principal components of the GYT biplot covered 88.3% of such a yield * trait variation. According to the GYT ranking relative to the ‘ideal’ genotype, which hypothetically should be located in the center of centric circles, the optimal combination of yield performance with tolerance to a set of abiotic and biotic stresses had accessions G94 Skald (POL), G96 Despina (DEU) and G93 Kormoran (POL). Accessions G85 Almonte (CAN), G95 Suveren (POL), G74 Dar Nosivshchyny (UKR), G81 Scarb (POL), G92 Vienna (AUT), G68 AC Alma (CAN), G75 Smarahd (UKR), G53 Tiver (UKR), and G54 Avers (UKR), which exceeded the standard G1 Vzirets (UKR) in terms of combination of yield performance with other adaptive traits, also have a high breeding value.
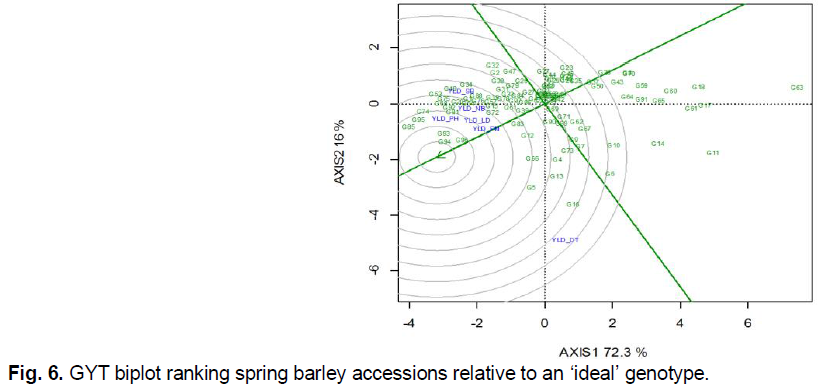
Fig 6. GYT biplot ranking spring barley accessions relative to an ‘ideal’ genotype.
Note: G1…G97 are codes for spring barley accessions accordingly to Table 1, YLD_DT is for yield and relative drought tolerance, YLD_LD is for yield and resistance to lodging, YLD_PM is for yield and resistance to powdery mildew, YLD_NB is for yield and resistance to net blotch, YLD_SB is for yield and resistance to spot blotch, YLD_PH is for yield and resistance to leaf rust.
Accessions G16 AC Alberte (CAN), G5 CDC Cartel (CAN), G13 AC Candle (CAN), G66 AC Westech (CAN), G4 Phoenix (CAN) exceeded the mean values for yield * trial combinations, but were shifted toward a combination of yield and relative tolerance to drought. Therefore, they are of practical interest as genetic sources for participation in hybridization to improve relative drought resistance.
Conclusion
In summary, significant differences were revealed in terms of yield performance and adaptive responses among the 96 spring barley accessions of different origin studied.
As a result of field trials and laboratory investigation, the new genetic sources in terms of high and stable grain yield, relative tolerance to drought, lodging resistance, and resistance to diseases have been identified. We have shown that according to the GYT ranking relative to the ‘ideal’ genotype, the optimal combination of yield performance with a set of adaptive traits had spring barley accessions Skald (POL), Despina (DEU), Kormoran (POL), Almonte (CAN), Suveren (POL), Dar Nosivshchyny (UKR), Scarb (POL), Vienna (AUT), AC Alma (CAN), Smarahd (UKR) Tiver (UKR), and Avers (UKR).
The new genetic sources identified are valuable in spring barley breeding to develop an initial material with combination of yield, its stability, and tolerance to the most widespread abiotic and biotic stresses under conditions of the central part of Ukrainian Forest-Steppe.
References
Abdel-Ghani, A.H., Sharma, R., Wabila, C., Dhanagond, S., Owais, S.J., Duwayri, M.A., Al-Dalain, S.A., Klukas, C., Chen, D., Lübberstedt, T., von Wirén, N., Graner, A., Kilian, B., Neumann, K. (2019). Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage. BMC Plant Biology, 19:216.
Arshadi, A., Karami, E., Sartip, A., Zare, M., Rezabakhsh, P. (2018). Genotypes performance in relation to drought tolerance in barley using multi-environment trials. Agronomy Research, 16:5-21.
Babayants, L.T., Mesterhazy, A, Wachter, F., Neklesa, N., Dubinina, L., Omel’chenko, L., Klechkovskaya, E., Slyusarenko, A., Bartosh, P. (1988). Methods of breeding and evaluation of wheat and barley resistance to diseases in the CMEA member countries. Prague, p:321 (in Russian).
Bengtsson, T., Åhman, I., Manninen, O., Reitan, L., Christerson, T., Jensen J.D., Krusell, L., Jahoor, A., Orabi, J. (2017). A novel QTL for powdery mildew resistance in Nordic spring barley (Hordeum vulgare L. ssp. vulgare) revealed by genome-wide association study. Frontiers in Plant Science.
Brantestam, A.K., Legziòa, L., Cristensen, T., Weibull, J., von Bothmer, R., Martynov, S., Yndgaard, F., Rashal, I. (2014). Characterisation of agronomic performance of Baltic spring barley material. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences, 68:119-132.
Clare, S.J., Wyatt, N.A, Brueggeman, R.S., Friesen, T.L. (2020). Research advances in the Pyrenophora teres–barley interaction. Molecular Plant Pathology, 21:272-288.
Comadran, J., Russell, J.R., Booth, A., Pswarayi, A., Ceccarelli, S., Grando, S., Stanca, A.M., Pecchioni, N., Akar, T., Al-Yassin, A., Benbelkacem, A., Ouabbou, H., Bort, J., van Eeuwijk, F.A., Thomas, W.T.B., Romagosa, I. (2011). Mixed model association scans of multi-environmental trial data reveal major loci controlling yield and yield related traits in Hordeum vulgare in Mediterranean environments. Theoretical and Applied Genetics, 122:1363-1373.
Darrier, B., Russell, J., Milner, S.G., Hedley, P.E., Shaw, P.D., Macaulay, M., Ramsay, L.D., Halpin, C., Mascher, M., Fleury, D.L., Langridge, P., Stein, N., Waugh, R. (2019). A comparison of mainstream genotyping platforms for the evaluation and use of barley genetic resources. Frontiers in Plant Science, 10:544.
Dawson, I.K., Russell, J., Powell, W., Steffenson, B., Thomas, W.T.B., Waugh, R. (2015). Barley: a translational model for adaptation to climate change. New Phytologist, 206:913-931.
Dinsa, T., Mekbib, F., Letta, T. (2018). Genetic variability, heritability and genetic advance of yield and yield related traits of food barley (Hordeum vulgare L.) genotypes in Mid Rift valley of Ethiopia. Advances in Crop Science and Technology, 6:401.
Dockter, C., Hansson, M. (2015). Improving barley culm robustness for secured crop yield in a changing climate. Journal of Experimental Botany, 66:3499-3509.
Dorofeev, V.F., Rudenko, M.I., Udachin, R.A. (1974). Drought-tolerant wheat (guidelines). Leningrad: VIR, p:186 (in Russian).
Dreiseitl, A. (2020). Specific resistance of barley to powdery mildew, its use and beyond: a concise critical review. Genes, 11:971.
Fazlikhani, L., Keilwagen, J., Kopahnke, D., Deising, H., Ordon, F., Perovic, D. (2019). High resolution mapping of RphMBR1012 conferring resistance to puccinia hordei in barley (Hordeum vulgare L.). Frontiers in Plant Science, 10:640.
Ferreira, J.R., Pereira, J.F., Turchetto, C., Minella, E., Consoli, L., Delatorre, C.A. (2016). Assessment of genetic diversity in Brazilian barley using SSR markers. Genetics and Molecular Biology, 39:86-96.
Filip, E., Russu, F., Porumb, I., Muntean, L., Ona, A., Boantă, A., Pamfil, D., Borza, G. (2020). Research regarding the approach of drought tolerance of two-row spring barley. AgroLife Scientific Journal, 9:127-131.
Gadissa, F., Abebe, M., Worku, B. (2021). Assessment on the current state of on-farm diversity and genetic erosion in barley (Hordeum vulgare L.) landraces from Bale highlands, Southeast Ethiopia. BioMed Research International.
Govindaraj, M., Vetriventhan, M., Srinivasan, M. (2015). Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genetics Research International, pp:431487.
Gudzenko, V., Vasylkivsky, S. (2016). Spring barley yielding capacity depending on hydrothermal conditions of cropping season in the Central Forest-steppe of Ukraine. Agrobiology, 2:11-17.
Hopkin, M. (2008). Biodiversity: frozen future. Nature, 452:404-405.
Hoseinzadeh, P., Zhou, R., Mascher, M., Himmelbach, A., Niks, R.E., Schweizer, P., Stein, N. (2019). High resolution genetic and physical mapping of a major powdery mildew resistance locus in barley. Frontiers in Plant Science, 10:146.
Hudzenko, V.M., Vasylkivskyi, S.P., Demydov, O.A., Polishchuk, T.P., Babiy, O.O. (2017). Spring barley breeding for increase in productive and adaptive capacities. Plant Breeding and Seed Production, 111:51-61.
Jabbari, M., Fakheri, B.A., Aghnoum, R., Nezhad, M.N., Ataei, R. (2018). GWAS analysis in spring barley (Hordeum vulgare L.) for morphological traits exposed to drought. PLoS ONE, 13:e0204952.
Jairus, O.J.S., Auma, E.O., Ngode, L. (2015). Evaluation of promising malting barley varieties using agronomic and quality traits in Kenya. Journal of Agriculture and Life Sciences, 2:104-110.
Koebner, R.M.D., Donini, P., Reeves, J.C., Cooke, R.J., Law, J.R. (2003). Temporal flux in the morphological and molecular diversity of UK barley. Theoretical and Applied Genetics, 106:550–558.
Laidig, F., Piepho, H.P., Rentel, D., Drobek, T., Meyer, U. (2017). Breeding progress, genotypic and environmental variation and correlation of quality traits in malting barley in German official variety trials between 1983 and 2015. Theoretical and Applied Genetics, 130:2411-2429.
Lillemo, M., Reitan, L., Bjørnstad, A. (2009). Increasing impact of plant breeding on barley yields in central Norway from 1946 to 2008. Plant Breeding, 129:484-490.
Lillemo, M., Orabi, J., Backes, G., Jahoor, A., Hermannsson, J., Christerson, T., Tuvesson, S., Gertsson, B., Reitan, L., Alsheikh, M., Aikasalo, R., Isolahti, M., Veteläinen, M., Jalli, M., Krusell, L., Hjortshøj, R.L., Eriksen, B., Bengtsson, T. (2019). Identification of ideal allele combinations for the adaptation of spring barley to northern latitudes. Frontiers in Plant Science, 10:542.
Malysheva-Otto, L., Ganal, M.W., Law, J.R., Reeves, J.C., Röder, M.S. (2007). Temporal trends of genetic diversity in European barley cultivars (Hordeum vulgare L.). Molecular Breeding, 20:309-322.
Megersa, G. (2014). Genetic erosion of barley in North Shewa zone of Oromiya Region, Ethiopia. International Journal of Biodiversity and Conservation, 6:280-289.
Mikołajczak, K., Kuczyńska, A., Krajewski, P., Sawikowska, A., Surma, M., Ogrodowicz, P., Adamski, T., Krystkowiak, K., Górny, A.G., Kempa, M., Szarejko, I., Guzy-Wróbelska, J., Gudyś, K. (2017). Quantitative trait loci for plant height in Maresi × CamB barley population and their associations with yield-related traits under different water regimes. Journal of Applied Genetics, 58:23-35.
Milner, S.G., Jost, M., Taketa, S., Mazуn, E.R., Himmelbach, A., Oppermann, M., Weise, S., Knüpffer, H., Basterrechea, M., König, P., Schüler, D., Sharma, R., Pasam, R.K., Rutten, T., Guo, G., Xu, D., Zhang, J., Herren, G., Müller, T., Krattinger, S.G., Keller, B., Jiang, Y., González, M.Y., Zhao, Y., Habekuß, A., Färber, S., Ordon, F., Lange, M., Börner, A., Graner, A., Reif, J.C., Scholz, U., Mascher, M., Stein, N. (2019). Genebank genomics highlights the diversity of a global barley collection. Nature Genetics, 51:319-326.
Ovesná, J., Kučera, L., Vaculová, K., Milotová, J., Snape, J., Wenzl, P., Huttner, E., Kilian, A., Martelli, G., Milella, L. (2013). Analysis of the genetic structure of a barley collection using DNA diversity array technology (DArT). Plant Molecular Biology Reporter, 31:280-288.
Pacheco, Á., Vargas, M, Alvarado, G., Rodríguez, F., Crossa, J., Burgueño, J. (2015). GEA-R (Genotype × environment analysis with R for Windows), Version 4.1, CIMMYT Research Data and Software Repository Network.
Panfilova, A., Mohylnytska, A., Gamayunova, V., Fedorchuk, M., Drobitko, A., Tyshchenko, S. (2020). Modeling the impact of weather and climatic conditions and nutrition variants on the yield of spring barley varieties (Hordeum vulgare L.). Agronomy Research, 18:1388-1403.
Pasam, R.K., Sharma, R., Walther, A., Özkan, H., Graner, A., Kilian, B. (2014). Genetic diversity and population structure in a legacy collection of spring barley landraces adapted to a wide range of climates. PLoS ONE, 9:e116164.
Poets, A.M., Mohammadi, M., Seth, K., Wang, H.Y., Kono, T.J., Fang, Z., Muehlbauer, G.J., Smith, K.P., Morrell, P.L. (2016). The effects of both recent and long-term selection and genetic drift are readily evident in North American barley breeding populations. G3 (Bethesda,) 6:609-622.
Pogoda, M., Liu, F., Douchkov, D., Djamei, A., Reif, J.C., Schweizer, P., Schulthess, A.W. (2020). Identification of novel genetic factors underlying the host-pathogen interaction between barley (Hordeum vulgare L.) and powdery mildew (Blumeria graminis f. sp. hordei). PLoS ONE, 15:e0235565.
Russell, J., Dawson, I.K., Flavell, A.J., Steffenson, B., Weltzien, E., Booth, A., Ceccarelli, S., Grando, S., Waugh, R. (2011). Analysis of >1000 single nucleotide polymorphisms in geographically matched samples of landrace and wild barley indicates secondary contact and chromosome-level differences in diversity around domestication genes. New Phytologist, 191:564-578.
Sayd, R.M., Amabile, R.F., Faleiro, F.G., Costa, M.C., Montalvão, A.P.L. (2019). Genetic parameters and agronomic characterization of elite barley accessions under irrigation in the Cerrado. Acta Scientiarum. Agronomy, 41:e42630.
Sato, K. (2020). History and future perspectives of barley genomics. DNA Research 27:1-8.
Statkevičiūtė, G., Leistrumaitė, A. (2010). Modern varieties of spring barley as a genetic resource for disease resistance breeding. Agronomy Research, 8:721-728.
Thabet, S.G., Moursi, Y.S., Karam, M.A., Graner, A., Alqudah, A.M. (2018). Genetic basis of drought tolerance during seed germination in barley. PLoS ONE, 13:e0206682.
van de Wouw, M., Kik, C., van Hintum, T., van Treuren, R., Visser, B. (2010). Genetic erosion in crops: concept, research results and challenges. Plant Genetic Resources, 8:1-15.
Vatter, T., Maurer, A., Perovic, D., Kopahnke, D., Pillen, K., Ordon, F. (2018). Identification of QTL conferring resistance to stripe rust (Puccinia striiformis f. sp. hordei) and leaf rust (Puccinia hordei) in barley using nested association mapping (NAM). PLoS ONE, 13:e0191666.
Visioni, A., Rehman, S., Vaish, S.S., Singh, S.P., Vishwakarma, R., Gyawali, S., Al-Abdallat, A.M., Verma, R.P.S. (2020). Genome wide association mapping of spot blotch resistance at seedling and adult plant stages in barley. Frontiers in Plant Science, 11:642.
Wehner, G., Balko, C., Humbeck, K., Zyprian, E., Ordon, F. (2016). Expression profiling of genes involved in drought stress and leaf senescence in juvenile barley. BMC Plant Biology, 16:3.
Yan, W., Frégeau-Reid, J. (2018). Genotype by yield*trait (GYT) biplot: a novel approach for genotype selection based on multiple traits. Scientific Reports, 8:8242.
Yan, W., Tinker, N.A. (2006). Biplot analysis of multi-environment trial data: principles and applications. Canadian Journal of Plant Science, 86:623-645.
Zargar, M., Bodner, G., Tumanyan, A., Tyutyuma, N., Plushikov, V., Pakina, E., Shcherbakova, N., Bayat, M. (2018). Productivity of various barley (Hordeum vulgare L.) cultivars under semi-arid conditions in southern Russia. Agronomy Research, 16:2242-2253.
Zhou, H., Muehlbauer, G., Steffenson, B. (2012). Population structure and linkage disequilibrium in elite barley breeding germplasm from the United States. Journal of Zhejiang University-Science B (Biomedicine & Biotechnology), 13:438-451.
Author Info
V.M. Hudzenko1*, O.A. Demydov1, T.P. Polishchuk1, I.V. Fedorenko1, A.A. Lysenko1, M.V. Fedorenko1, A.A. Siroshtan1, T.V. Yurchenko1 and T.V. Shevchenko22National Academy of Agrarian Sciences of Ukraine, Presidium, Kyiv, Ukraine
Citation: Hudzenko, V.M., Demydov, O.A., Polishchuk, T.P., Fedorenko, I.V., Lysenko, A.A., Fedorenko, M.V., Siroshtan, A.A., Yurchenko, T.V., Shevchenko, T.V. (2021). Comprehensive evaluation of spring barley yield and tolerance to abiotic and biotic stresses. Ukrainian Journal of Ecology 11 (8), 48-55.
Received: 18-Sep-2021 Accepted: 07-Oct-2021 Published: 23-Oct-2021
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
