Research - (2022) Volume 12, Issue 11
Comparative physico-chemical studies between tamersite and djaarir springs from Khenchela region (Northeastern Algeria)
S. Guilal1, Y. Nedjar2 and S. Lakhdari3*Abstract
North-eastern Algeria is characterised by several thermal springs. The aim of this work is to study the Tamersite spring, which has not received any study and to compare to those relating to the Djaarir spring. For this purpose, four samples were collected from these thermal springs in 2017, having a temperature ranging between 26 and 35°C, anal most neutral pH and high values of electrical conductivity. In order to evaluate the thermal water potential of these springs, we adopted an interdisciplinary approach. The hydrochemical tool shows that hot water is characterised by the presence of two different chemical facies: the first of bicarbonate–sodium type, the second of chloride-sodium type. This analysis allowed also attributing a terriferous, evaporitic origin to sodium, chlorides and bicarbonates. The salinity characterizing these thermal waters is mainly due to deep runoff in cristallophyllian formations and carbonates formations being in contact with the terriferous, saliferous formations.
Keywords
Algeria, Thermal waters, Tamersite spring, Djaarir spring, Hydrochemistry, Chemical facies.
Introduction
The valorization of thermal water and the development of both curative and ludic thermalism need today a thorough knowledge of the origin and the available amount of water, without the hazard of affecting the quality of this resource. The geothermal fields are largely investigated in order to understand the mechanisms of hot springs, and that is why a number of countries conducted researches on the exploitation of geothermal resources in the course of the last decades. Chemistry of thermal water was the focus of several studies; most of which were concerned about two aspects:
The interaction between thermal water and the wall rocks has a major control on the chemical property of thermal water (ÜGemici et al., 2002; Cruz et Franca, 2006), and the origin of geothermal water can be estimated with the help of chemical geothermometry (B Houha, 2007; Ben Abidate, 1998).
Among the Algerian thermo-mineral heritage consisted of more than 200 thermal springs indexed, located in different areas with complex geological structure (J Polvêche, 1960) this number increases regularly by moving eastward, we choosed two thermal springs in the Khenchela area; they are concerned by this work. The regional geology is marked by sedimentary rocks inherited from the marine transgressions of Secondary and Tertiary (B Houha, 2007). The hydrothermal systems can be found in many geological settings and are hosted in different types of wall rocks, but they are often found in the areas of thermal and volcanic flow associated with tectonic plates limits and/or in areas with high porosity and permeability. Water in the sediments is heated by the flow of the regional heat. The study area in north-eastern Algeria appears as a large landscape slightly undulated, stretching between the Saharan Atlas in the south, the Mediterranean in the north and the Tunisian boundaries in the east (Fig. 1).
Fig 1: Geographical location of the study area.
The present study is concerned about the chemical analyses of two thermal springs of north-eastern Algeria in order to understand the origin, to determine the chemical processes governing the composition and the movement of these fluid giving rise to salinity of these latter and to estimate temperatures of the subsurface reservoir.
Four samples of thermal water were taken during April 2017. For this purpose, field and laboratory analyses were performed. Temperature, pH and conductivity of spring water were directly measured in situ by means of a portable device, whereas the hydrodynamic properties were analyzed at the laboratory. The thermal springs are frequently developed into thermal spa sand baths. They are also used for curative purposes and for improving the social and economic welfare of the population.
Materials and Methods
Geographical location of the thermal springs
a. Hamam TAMERSITE
The Tamersite spring is located in the El Ouldja municipality south-west of the Khenchela province in the Aures in eastern Algeria, and stretches over a surface area of 366 km2. It is limited to the north by El M’Sara, to the east by Kheirane, to the south by the Biskra province and to the west by the Batna province.
In a woody area, with an adequate climate and relief diversity, planted with Palma olive trees on the banks of the El Arab wadi, the first spring (Tamersite) is located on the right side of the runoff direction of a wadi. We have observed the discharge of this spring on the left side of the wadi located 19 meters far from the first spring. This spring is divided into two springs: one called Tamersite 1 and the other one called Tamersite 2 located 1.19 kilometer far from the first spring.
b. Hamam DJAARIR
The hamam Djaarir spring is located 7.43 kilometers far from the Bouhmama locality, south-west of the Khenchela province in the Aures in eastern Algeria and stretches over a surface area of one hectare. This spring is divided into two springs: H. Djaarir 1 and H. Djaarir 2 that are close to each other over a distance of 90 m. From the climatic point of view, this is an arid-climate area.
The annual rainfall recorded at the main stations of the study area is marked by a temporal variability. It varies between a mean of 144 and 170 mm. We observe a rainfall deficit of 15%, thereby giving rise to a decrease in depth of runoff by near 53% which is reflected by a decrease in supply by 32%. The annual mean temperature is of 20°C.
The drainage network is temporary (ephemeral runoff) and little developed.
The configuration of this system can be explained by the rains scarcity on the one hand and by the topographical nature of the area on the other hand. So, the study area is essentially crossed by El Arab wadi, which is the natural drain and the valley collector, and which runs from north to south. The catchment surface area of the El Ouldja impoundment is of 1333 km²; it is marked by a thalweg length of 113 km and a total slope of 1.36%.
The two springs emerging in two different sub basins, the main streams of which meet further downstream; they are separated by a hill (Fig. 2).
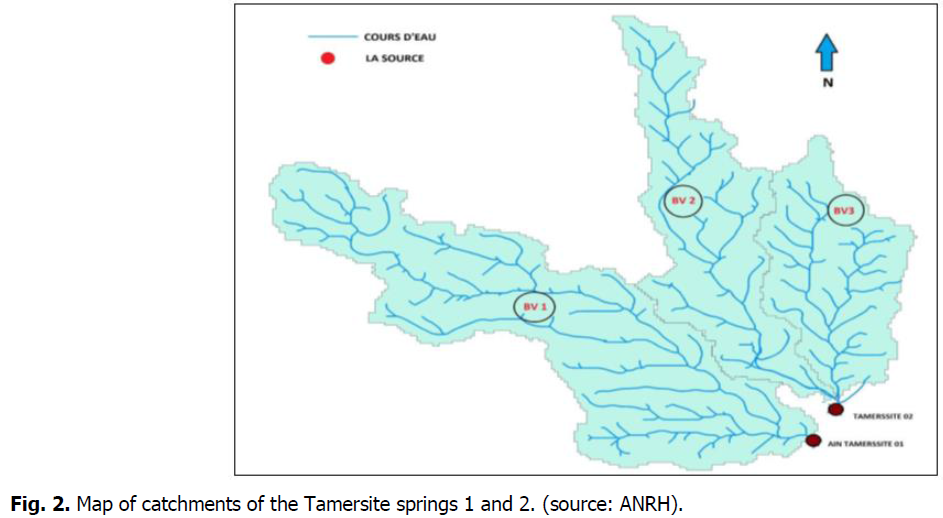
Fig 2: Map of catchments of the Tamersite springs 1 and 2. (source: ANRH).
Geologically, the first one belongs to Kheirane, the findings of which were exploited for the Tamersite springs. The findings revealed the significance of the Maastrichtian limestone reservoir next to sandstone Miocene and Quaternary formations.
The second site, hamam Djaarir, indicates the significance of Miocene sandstone reservoir as well as that of Pliocene essentially made up of conglomerates; it is often very difficult to differentiate this level from the Quaternary formations. It is exploited in the southern Aures and has high quality water. The underlying formations consist of marl-limestone alternations.
The discharge of the Tamersite spring 1 lies at limestone of Cenomanian age having a dip of 10°C, with a direction N110E on the right bank of a wadi beneath a fairly large thickness of marl formations of the same age, with limestone centimetric bars.
The Tamersite spring 2 is located beneath a limestone flagstone outcropping at a wadi. The overall description is as follows:
At the base (at the wadi) there are limestone beds having black color with a trace of a tectonic activity revealing striate and solifluction niches.
Going up, limestone becomes clearer with a presence of blackish marl with layer alternating with these latter.
These marls passages do not exceed in the best cases 50 cm.
The right bank exhibits a marl formation.
The area of the two springs hamam Djaarir 1 and 2 is located in a depression surrounded by reliefson the northern and western side; we know especially the Dj.Chelia. It is covered by recent formations. The gulling son the side of the spring 2 reveal marls with micritic black limestone nodules surmounted by a recent layer of Quaternary, with pebbles dispersed in a marl matrix (Fig. 3).
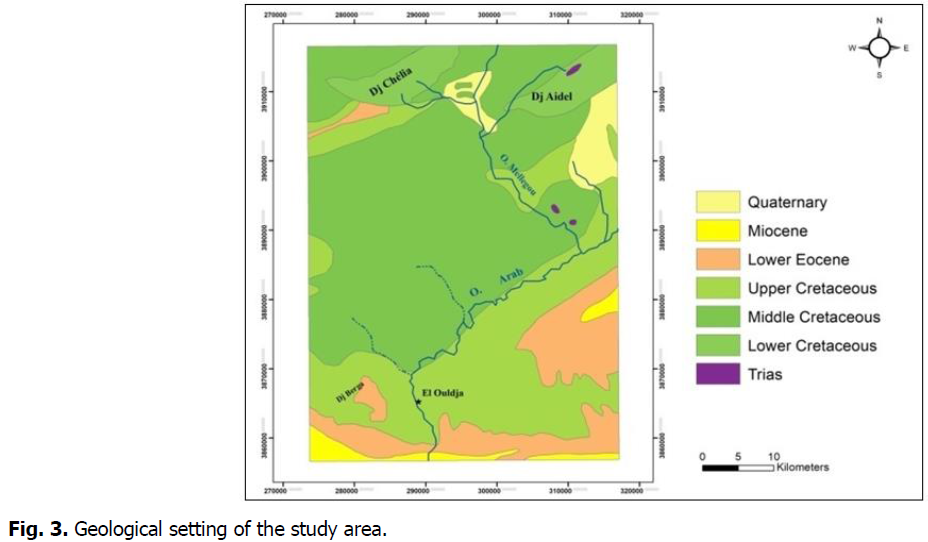
Fig 3: Geological setting of the study area.
Particularly critical for thermal waters is the need to identify the source of the spring and to sample as close to the spring as possible (IAEA 2008).
The sampling focused on the two Tamersite springs (Tamersite 1 and 2) and two other ones of hamam Djaarir (Djaarir 1 and 2) (Fig. 4), Chemical analyses were carried out at le Laboratoire de l’Agence Nationale des Ressources Hydriques (ANRH) of Khenchela during April 2016.
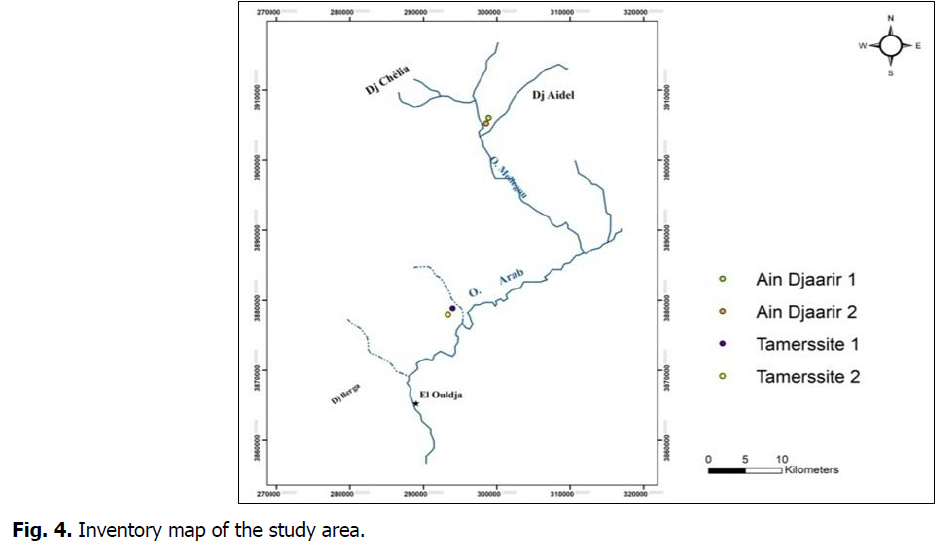
Fig 4: Inventory map of the study area.
Strict requirements were met during the whole period of sampling according to Rodier recommendations 2009. The samples were carried to the laboratory in a cool box and are then stored in a refrigerator until the analysis.
As regards the sensitive parameters such as temperature, electrical conductivity (EC) and pH they were measured in situ using a multi-parameter probe. The chemical elements analyzed are: calcium (Ca2+), magnesium (Mg+2), sodium (Na+), potassium (K+), chloride (Cl-), bicarbonate (HCO3-), sulfate (SO4-2), nitrate (NO3-), silica (SiO2), cadmium (Cd+2), nickel (Ni+2), barium (Ba+2), lithium (Li+), aluminum (Al+3), lead (Pb2+), iron (Fe) and strontium (Sr2+). Three methods of analysis have been use to determine the concentrations of different chemical elements. The determination of the content of bicarbonates and chlorides was done by titrimetry. The cations including the elements were determined using a Perkin Elmer, 1100B flame atomic absorption and by titration potentiometric as proposed by RODIER (1996). Nitrates were determined according to the technique recommended by the Center of Expertise in Environmental Analysis of Quebec (CEAEQ 2008). The results of the analyzes have been reported in Table 1.
| Springs | T° | ph | c25°C | O2 | TDS | Ca | Mg | Na | K | CL | SO4 | HCO3 | NO3 | NH4 | SiO2 | Zn | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tamrsit 1 | 27.04 | 7.64 | 200 | 4.6 | 920 | 48.1 | 33.05 | 619.23 | 146 | 20.29 | 54.2 | 727.22 | 1 | 0 | 18.6 | 0.0067 | 0.0064 | 0.0083 |
| Tamrsit 2 | 25.5 | 7.6 | 1691 | 6.7 | 813 | 57.72 | 21.87 | 452 | 99.5 | 13.52 | 1.89 | 534.65 | 0 | 0.37 | 18.2 | 0.0038 | 0.1551 | 0.0850 |
| A.Djarir 1 | 34 | 7.49 | 5350 | 7.2 | 2670 | 234.07 | 28.19 | 1374.15 | 166 | 1710.76 | 287.55 | 222.47 | 1 | 0.14 | 26.3 | 0 | 0.01721 | 0 |
| A.Djarir 2 | 30.7 | 7.46 | 5360 | 5.2 | 2680 | 238.88 | 21.87 | 1450.68 | 148 | 1710.76 | 262.46 | 200.51 | 1 | 0.24 | 27.8 | 0.0165 | 0.0214 | 0.2372 |
Table 1. Physico-chemical composition of sampled watersa.
The chemical facies of groundwater were determined from the Piper diagram using a computer software developed by Simler, 2014 and AquaChem 2014. 2 for the ternary diagrams. We make use of GeoTcode that uses as entry the chemical composition of water and the saturation indices of minerals to compute the water-rock balance on a temperature.
Results and Discussion
Physicochemical parameters
The in-situ parameters are reported in Table 1. The temperatures of thermal waters are between 29°C measured in spring of Hammam TAMERSITE and 35°C in the Hammam DGAARIR. The different outlet temperatures of the thermal springs are attributed to local conditions depends on the velocity of water flow, time of circulation and fracture characteristics (H Chenaker, et al., 2017), The high temperature of a spring depends on both the depth and the upwelling speed (R Cidu et al., 2008). The temperature of the thermal spring thus reflects the depth of penetration of the water and the rate at which it ascends to the surface (E Grasby et al., 2001).
The lowest temperatures are due to cooling caused by mixing between the deep thermal waters and the shallow meteoric waters and may be due to greater meteoric recharge or greater heat conduction due to shallow reservoir depths (H Chenaker, et al., 2017).
This thermal imbalance between the atmosphere and the aquifer indicates that the waters have a very deep origin. They are influenced by the geothermal gradient on the one hand, seismic activity, radioactive decay and endogenous chemical reactions producing energy on the other hand. Note also that the friction between the geological layers during the seismic activity produces heat that heats the water of the aquifers (Ch Berkani, et al., 2017).
The pH values of these springs vary from slightly acidic to slightly alkaline. The maximum value of pH was recorded as 7.8 at (HM M) Hammam TAMERSSITE and the minimum value of pH was recorded as 7.4 at (HS K) Hammam DJAARIR. As per the findings, it was observed that the water was slight alkaline in nature which might be due to the presence of the dissolved salts in the water (H Chenaker et al., 2017).
The electrical conductivity was found to be quite high, the maximum value of conductivity measured at temperature of sample was recorded as 5360 S/cm at Hammam DJAARIR and the minimum value was recorded as 1691 S/cm at Hammam TAMERSSITE. Due to the presence of high amounts of minerals in the water and many salts (NaCl-, CaSO4-2 H2O, CaSO4- and Na2+SOH-) are more soluble at higher temperatures, these salts and minerals enter the water from rocks and sediment in contact with it, the hot springs showed higher conductivity value related to the high total dissolved solids (TDS) (H Chenaker et al., 2017; Ch Berkani et al., 2017).
The mineralization of the waters is determined by the chemical and mineralogical nature of the sediments they flew through. The thermo-mineral waters are much mineralized. They are directly related to the gypsum-saline sediments of Triassic so widespread in Algeria, To better understand the process of mineralization of thermal waters, it is necessary to represent the major elements as a function of chloride (B Houha, 2007). The latter is a conserved element, does not participate in water-rock interactions, characterizes the origin of the salinity of the waters and constitutes a tracer of mixture. They are all more than 2400 mg/l.
Water chemistry
Piper diagram
The Physico-chemical properties of thermal water depend on the composition of infiltrating solution, migration depth, residence time of thermal water in the travel pathway and water-rock interaction in deep formations. The physico-chemical properties of water are linked to its subsurface path line, temperature depth, nature of crossed rocks and temporary residence. The thermal springs have a variety of chemical types; this variability is due to lithological composition of sampling sites, location on several different geological structures and complexes (J Polvêche, 1960). The different water samples were classified according to their chemical composition with the help of Piper diagram (J Piper, 1944) and the software diagram. This diagram (Fig. 5) shows that the overall chemical characteristic lies in the following two water types:

Fig 5: Piper diagram showing the chemical facies.
For the Tamersite site springs (Figure below), carbonates (anions) and sodium (cations) are largely dominant over other elements owing to limestone leaching by hot water during its rise to the land surface. The low value of sulfates for Tamersite 2 may be explained by the involvement of modifying phenomena (sulphur-reducing bacteria which decreases the sulfates content) (T Kompani-Zare et al., 2001) his projection of analyses findings on Piper diagrams (anions-cations) corroborates the dominances already described and shows that:
The Tamersite springs lie on the side of carbonate pole with regard to anions and sodium pole in the cations triangle. In fact, these springs are classified as bicarbonate-sodium facies.
As regards water of the Djaarir springs the points in the anions triangle lie on the side of chlorides, but for cations it is always sodium that dominates; the typical, chemical facies of this water will thus be chloride-sodium facies.
On the whole, all these waters originate from sodium-chloride water as a result of the physical environment, or the reactions with wall rocks and water-rock interaction (E Grasby et al., 2001) in the sampling area as well as the reactions close to thermal water surface with less deep saltwater-still water associated with Tertiary rocks of NaCl- type probably owing to dissolution of Triassic halite. According to Piper diagram it seems that the composition of seeping shallow water is influenced by halite layers.
Durov diagram
The diagram of Durov, built on the basis of the relative contents (meq/l) of major ions in solution allows visualising the distribution of sample compositions. The latter has for axes of braided two diagrams of Piper, respectively anions and cations. Thus, for every sample, we represent its composition in cations and anions on the corresponding axis. The intersection of the projection of the two coordinates obtained gives the representative point of the sample in the diagram of Durov. This diagram allows to distinguish easily the samples of (Na+-Ca++)-HCO3-from those of type (Na+-Ca++)-Cl-, respectively named the type bicarbonated and the chlorinated type. This distinction is often used on the literature to distinguish groundwaters, the signature of which gets closer to those to high salinity (chlorinated type), to those whose signature gets closer to some water of refill (type bicarbonated) (Mr Gascoyne et al., 1994; J Toth 1999) (Fig. 6).
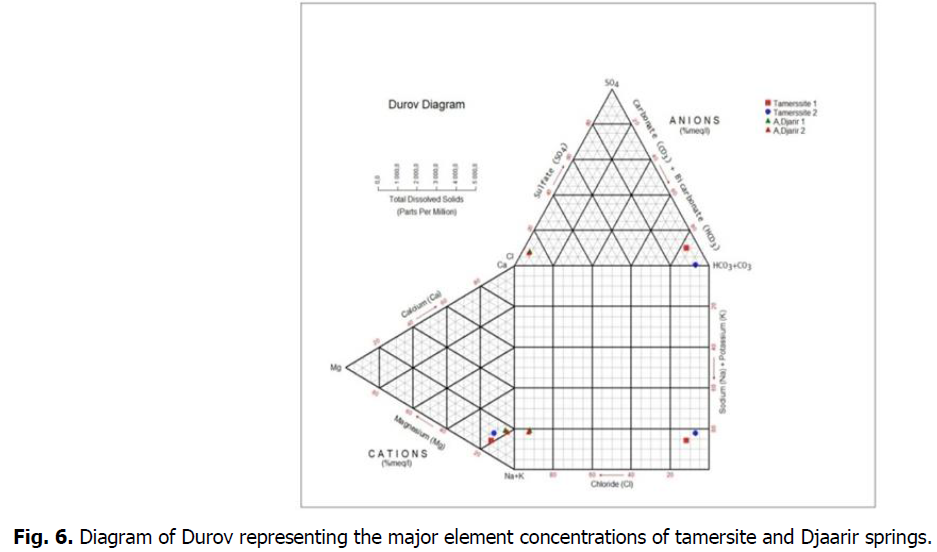
Fig 6: Diagram of Durov representing the major element concentrations of tamersite and Djaarir springs.
The projection of findings of water chemical analyses on Schoeller diagram (Fig. 7) shows that:
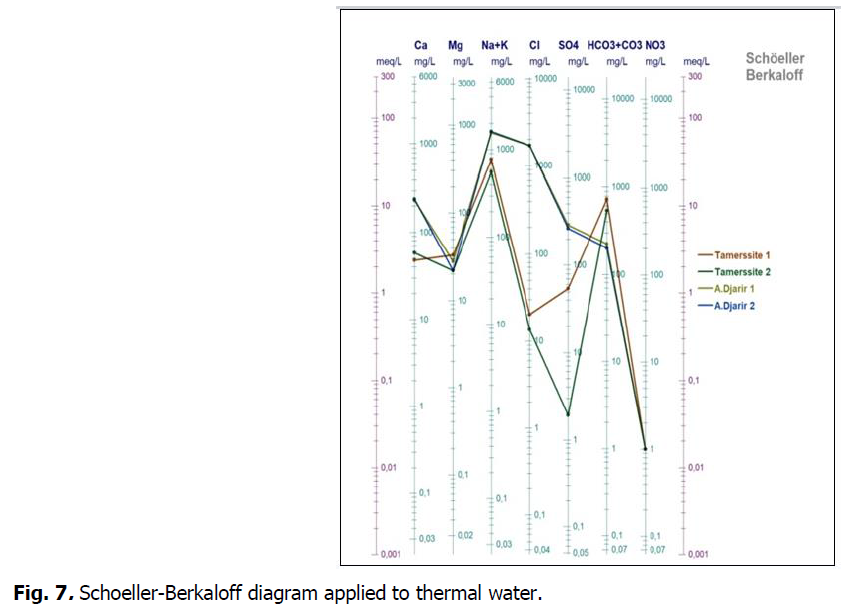
Fig 7: Schoeller-Berkaloff diagram applied to thermal water.
The broken lines relating to the springs are parallel with respect to the same site and intersect when the sites are different (parallel lines, so waters of the same family correspond to different origins when intersecting).
Geothermometry
Geothermometry is a tool enabling to evaluate the depth of geothermal reservoirs and temperature of the last chemical or isotopic balances prior to discharge. Deep water and gas move to the land surface and generally carry their geochemical history with them.
However, during its rise, hot water may undergo a mix with cold surface water, which may complicate the use of chemical geothermometers, thus leading either to an overestimation or an underestimation of temperatures. It is to be noted that the rise of thermal water from its genuine reservoirs is usually accompanied by temperature decrease and change in total mineralization (S Bouri et al., 2007).
The use of geothermometers suggests that there is no significant chemical change in water during its rise, despite the different, possible and usually remarkable cooling’s. In order to estimate temperature of the last thermodynamic balance, we made use of many geothermometers, but it seems that that of silica (quartz) is the best suited to thermal water.
In the two cases, we see a difference between temperature of deep water and that of 32 to 42°C vent, which may be explained by a dissipation of energy during water flow from the reservoir to the land surface. This dissipation could be due either to a mix with surface water or to thermal diffusion associated with the long distance travelled by this water to reach the land surface. The K‑Na‑Mg 1/2 Giggenbach triangular diagram (1988) (Fig. 8) revealed that the thermal water samples correspond to immature water and is rather close to Mg corner, thereby indicating that this water did not reach its full balance. The rise of immature water (shallow water/mixed water) from deep geothermal reservoirs, with a certain degree of confidence, may be due to reactions with wall rocks during the rise from the reservoir. This geothermal tool allowed also evaluating the depth of the different reservoirs according to (H. Boucharebha et al., 1994; A Issaadi 1996; A Issaadi et al., 1997).
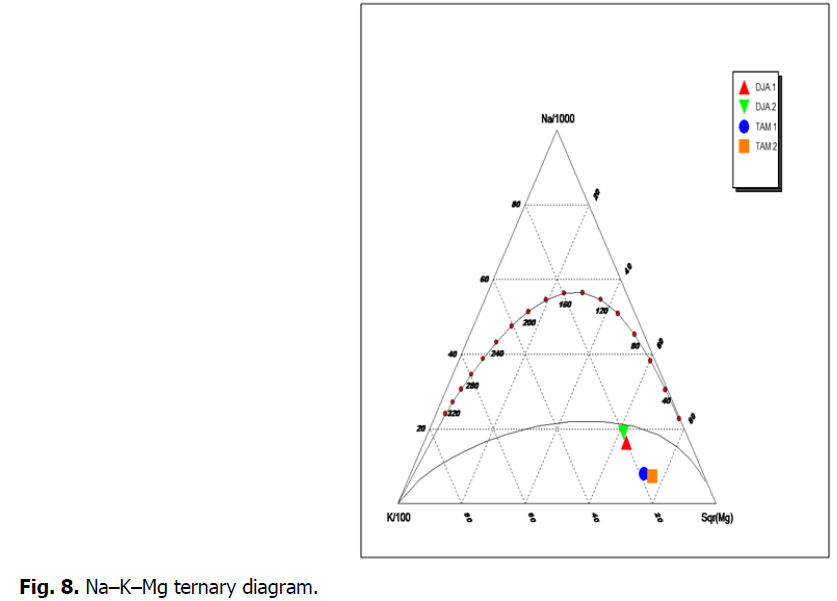
Fig 8: Na–K–Mg ternary diagram.
State of mineral sand other parameters in water
Saturation index
Balance of water with the matrix is of ten expressed by the saturation index:
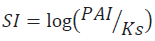
Where PAI is the activity product of concerned ions, Ks is the product of dissolubility of the considered mineral (S Bouhlassa, et al., 2008). The saturation degree, the sub saturation or the state of balance of a solution toward a mineral is only appreciable if the solubility product can be compared to the ionic activity product of referential ions in solution (A Droubi et al., 1978). A zero saturation index does not mean that water is in balance with the studied mineral. Water will be undersaturated if SI<0 (mineral dissolution) and saturated if SI<0 (mineral precipitation) (F Fekrache 2015).
The simulation of studied springs waters by natural evaporation was carried out using the thermodynamic software «Phreeqci 5.2» under the effect of isotherm evaporation (25°C). This simulation allowed computing the saturation indices (SI) and addressing the speciation of minerals.
The graph below illustrates the position of the different parameters and minerals with respect to the balance with studied waters.
We note that waters of Tamersite springs 1 and 2 are sharply undersaturated (moderately loaded water) with respect to evaporitic minerals such as halite, gypsum and anhydrite, whereas those of hamam Djaarir lie close to the balance (very loaded water).
For the carbonate minerals such as calcite, dolomite and aragonite, waters of the two sites (Tamersite and Djaarir) lie close to the balance to slightly supersaturate. In contrast, dolomite exhibits a different behavior. For the Tamersite springs, waters are saturated, whereas for the Djaarir springs, waters appear to be in balance with this mineral.
Waters come from depths where the medium is reducing (poor in O2) and undergo a pressure drop at the surface,which allows to carbon dioxide to escape (Fig. 9).
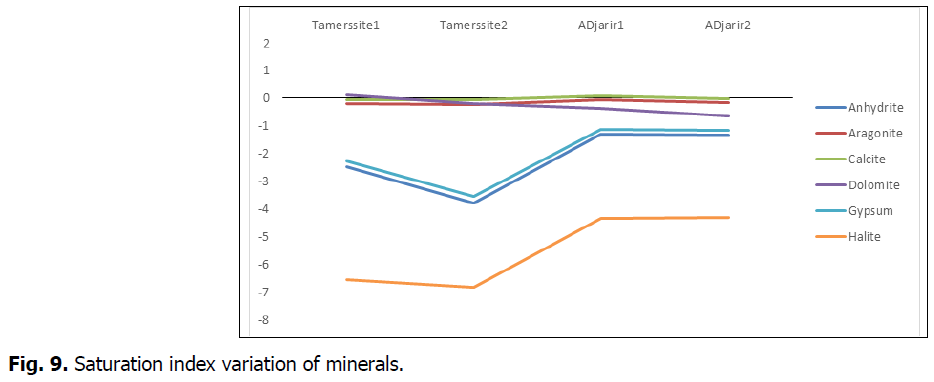
Fig 9: Saturation index variation of minerals.
Probable origins of the studied springs
The aquifers are already exploited everywhere in the area and water is cold in natural state. The springs are thought to come from depths below these aquifers. Their discharge to land surface is caused by many faults affecting the area especially the Dj. Chelia strike-slip fault. All this support the assumption that the very loaded springs waters studied do not originate from these aquifers, but rather from deeper zones. Meteoric water moves through the layers to reach the depths and becomes hot under the geothermal gradient and resume sits rise to the land surface through the disturbances (faults…). During its migration (downward or upward) it moves through the geological formations and thus acquires their geochemical signature and is more loaded with dissolved salts.
Djarir springs
The Djaarir springs 1 and 2 are located at the Chelia thrust; this latter reaches the Triassic formations in depth.
The chemical analyses carried out on these springs provide temperature values of (30°C) as well as very high chloride, sodium and potassium concentrations (Cl: 1689 mg/l-Na: 1438 mg/l-: 162 mg/l) unlike water of the aquifers exploited in the area.
The identical chemical compositions of waters of the Djaarir springs 1 and 2 suggest that this is the same spring shared under ground to give two springs,and allow assuming that the mineralization is the result of the contact of these waters in depth with the Triassic (evaporitic: gypsiferous and saliferous) formations. The high chlorides, sodium and potassium concentrations confirm this assumption. The Triassic formations being plastic they all follow the faults and form intrusions.
The hot springs of the Khenchela area, namely, the Hammam Essalhine and the Hammam Knif springs both exhibit similar (sodium-chloride) facies as the Djaarir springs do. It seems that those springs follow the same mechanism of temperature and mineralization acquisition whereas the pathways toward the land surface are distinct.
Tamersite springs 1and 2
The Tamersite springs emerge in limestone in contact with marl (impervious rocks).
In fact, we distinguish three springs at this site; two are close (on either side of a wadi) and the other is not far.
The waters chemical composition of these springs (1 and 2) is not fully identical although they have the same (bicarbonate-sodium) facies.
The bicarbonates concentration is very high (720 and 531 mg/l) far from that of chlorides (18 and 10 mg/l) and sulfates (53 and 1, 9 mg/l). For the cations sodium (620 and 460 mg/l) it is largely higher than calcium (48 and 57 mg/l) and magnesium (31 and 20 mg/l). Potassium in contrast is very significant (140 and 96 mg/l). These fairly excessive concentrations cannot originate from the aquifers of the area. Their origins are certainly deeper.
These origins seem to be linked to several assumptions:
The first one, the rocks dissolution that may generate high-concentration bicarbonates and sodium/potassium for example (Na2 (CO3) • 10 H2O contents).
The second assumption could be linked to the manifestations of like age abnormalities; this supposes the presence of boiling point water reservoir and an efficient (impervious cover). This phenomenon was studied by J. Facca (GEMP); 1967 during the geothermal investigations performed on the springs of the Bounamoussa dam area (Annaba). These springs exhibit the same temperature (30°C) as well as the same facies as those of the Tamersite springs. The question which remains to be asked: why is temperature average and does not reach that of Hammam Essalhine in Khenchela for instance not far from these springs? As an answer we think that, during their rise, the studied springs waters may be mixed (at the land surface or in average depth) with the aquifers waters of the area. The mixture is not total since the mineral load is not similar, and so each water has its own density.
Conclusion
The adopted methodology revealed that the 04 samples collected from the hot springs show that: La nappe d’eau est profonde d’environ 900 m, elle seront dite eaux superficiel, hypothermales, alcalines, d’origines probablement karstique.
Chemically: Tamersite spring 1,2: pure water mainly bicarbonate-sodium and secondary sulfates-calcique facies, moderately ionized. Djaarir spring 1,2; hard water mainly chlorides sodiques and secondary sulfates calcique,richly ionized which corroborates the influence of the geological formations, dominated by the Tertiary carbonate and evaporated rocks owing to the variability of the lithological composition and to different hydrogeological systems. While attempting to know the state of fluid-mineral balance using the saturation index (SI), we remarked that waters of the Tamersite springs 1 and 2 are dramatically undersaturated relative to evaporitic minerals, namely, halite, while those of hamam Djaarir relatively lie close to balance. The chemical analyses performed on these springs provide temperature values (30°C) well as high chlorides, sodium and potassium concentrations unlike the aquifers waters exploited in the area. The hot springs of the Khenchela area, namely, the Hammam Essalhine and the Hammam Knif springs both exhibit similar (sodium-chloride) facies as the Djaarir springs do. It seems that those springs follow the same mechanism of temperature and mineralization acquisition whereas the pathways differ. The fairly excessive cations and anions concentrations of the Tamersite springs cannot originate from the aquifers of the area. Their origins are certainly deep. These origins seem to be connected to rocks dissolution that may generate high-concentration bicarbonates, sodium and potassium.
References
Berkani, C., Houha, B. (2017). Physico-chemical and therapeutic characteristics of the thermo-mineral waters of Khenchela region (Northeastern Algeria). Journal of Material and Environmental Science, 8:1546-1553.
Bouri, S., Gasmi, M., Jaouadi, M., Souissi, I., Mimi, A.L., Dhia, H.B. (2007). Integrated study of surface and subsurface data for prospecting hydrogeothermal basins: case of the Maknassy basin (central Tunisia). Hydrological Sciences Journal, 52:1298-1315.
Bouchareb-Haouchine, F.Z., Issaad, A., Bendhia, H. (1994). Estimation and interpretation of geothermal gradient in Northern Algeria. Bulletin Service of Geology. Algeria, 5:69-74.
Bouchareb-Haouchine, F.Z., Boudoukha, A. (2009). Hydrogeochemical and lithostructural approach of deep circulations in the mounts of Hodna Algeria. European Journal of Scientific Research.
Bouhlassa, S., Alechcheikh, C., Kabiri, L. (2008). Origine de la minéralisation et de la détérioration de la qualité des eaux souterraines de la nappe phréatique du Quaternaire du bassin-versant de Rheris (Errachidia, Maroc). Science et Changements Planétaires/Sécheresse, 19:67-75.
Cruz, J.V., França, Z. (2006). Hydrogeochemistry of thermal and mineral water springs of the Azores archipelago (Portugal). Journal of Volcanology and Geothermal Research, 151:382-398.
Chenaker, H., Houha, B., Valles, V. (2017). Isotope studies and chemical investigations of hot springs from North-Eastern Algeria. Journal of Material and Environment Science, 8:4253-4263.
Al-Droubi, A., Fritz, B., Gac, J.Y., Tardy, Y. (1980). Generalized residual alkalinity concept; application to prediction of the chemical evolution of natural waters by evaporation. American Journal of Science, 280:560-572.
Fekrache, F. (2015). Contribution à l’étude de l’origine de la salinité des eaux du lac Fetzara-Annaba (Algérie).
Grasby, S.E., Hutcheon, I. (2001). Controls on the distribution of thermal springs in the southern Canadian Cordillera. Canadian Journal of Earth Sciences, 38:427-440.
Gemici, Ü., Tarcan, G. (2002). Hydrogeochemistry of the Simav geothermal field, western Anatolia, Turkey. Journal of Volcanology and Geothermal Research, 116:215-233.
Han, D.M., Liang, X., Jin, M.G., Currell, M.J., Song, X.F., Liu, C.M. (2010). Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. Journal of Volcanology and Geothermal Research, 189:92-104.
Houha, B. (2007). Etude du fonctionnement hydrogéologique et salin d'un bassin semi-aride. Rémila-Khenchela.
Bouchareb, H.F.Z. (1993). Apport de la géothermométrie et des données de forages profonds à l’identification des ressources géothermiques de l’Algérie du Nord. Application à la région du Hodna. Mémoire de Magister, Universal Alger, Algérie, p:105.
Centre d’expertise en Analyse Environnementale du Québec. (2008). Détermination des nitrates et des nitrites: méthode colorimétrique automatisée avec le sulfate d’hydrazine et le n.e.d. Ma, 3:300.
IAEA. (2008). Manual for operation of an isotope. Hydrology Laboratory, 10:167-178.
Issaadi, A. (1996). Mechanisms of operation of hydrothermal systems. Application to Algerian thermomineral waters and Hammam Bou-Hadjar waters. Bulletin du Service Géologique de l Algérie, 7:71-85.
Issaadi, A., Bouchareb-Haouchine, F.Z. (1997). Estimation du flux de chaleur en Algérie du Nord à partir de la thermométrie silice. Bulletin du Service Géologique de l Algérie, 8:29-39.
Issaadi, A. (1992). Le thermalisme dans son cadre géostructural, apports à la connaissance de la structure profonde de l’Algérie et de ses ressources géothermales.
Polvêche, J. (1960). Contribution à l’étude géologique de l’Ouarsenis Oranais Tome I et II. Bulletin.
Piper, A.M. (1944). A graphic procedure in the geochemical interpretation of water‐analyses. Eos, Transactions American Geophysical Union, 25:914-928.
Rodier, J., Geoffray, C., Rodi, L. (1984). L'analyse de l'eau: eaux naturelles, eaux résiduaires, eau de mer: Chimie, physico-chimie, bactériologie, biologie.
Vincent, V. (2008). Course on the thermal waters. University of Avignon, France.
Kompani-Zare, M., Moore, F. (2001). Chemical thermometry and origin of the Dalaki mineral springs, Bushehr Province, Iran. Journal of Hydrology, pp:189-204.
Gascoyne, M., Kamineni, D.C. (1994). The hydrogeochemistry of fractured plutonic rocks in the Canadian Shield. Applied Hydrogeology, 2:43-49.
Toth, J. (1999). Groundwater have has geologic agent: the overview of the Year talk, process, and demonstrations. Hydrogeology Journal, 7:1-14.
Author Info
S. Guilal1, Y. Nedjar2 and S. Lakhdari3*2Department of Nature and Life Sciences, Faculty of Exact Sciences and Nature and Life Sciences, University of Larbi Ben; hidi, Oum-El-Bouaghi, Algeria
3Laboratory of Biotechnology, Water, Environment and Health, University of Abbes Laghrour, Khenchela 40000, Algeria
Citation: Guilal, S., Nedjar, Y., Lakhdari, S. (2022). Comparative physico-chemical studies between tamersite and djaarir springs from Khenchela region (Northeastern Algeria). Ukrainian Journal of Ecology. 12:1-11.
Received: 26-Oct-2022, Manuscript No. UJE-22-78208; , Pre QC No. P-78208; Editor assigned: 27-Oct-2022, Pre QC No. P-78208; Reviewed: 08-Nov-2022, QC No. Q-78208; Revised: 12-Nov-2022, Manuscript No. R-78208; Published: 17-Nov-2022, DOI: 10.15421/2022_408
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
