Research Article - (2021) Volume 11, Issue 3
Comparative morphology of scent glands in marals (Cervus elaphus sibiricus, Severtzov, 1872)
N.D. Ovcharenko*, E.A. Kuchina and A.V. MatsyuraAbstract
The morphological parameters of the scent skin glands of red deer from 20 adult males inhabiting park conditions in the Altai Krai (Siberia, Russian Federation) were studied. The preorbital and meibomian glands on animal heads and the caudal glands on the torso were investigated. Classical histological and statistical methods of investigation were used. Seasonal dynamics of morphological equivalents of the functional state of the glands were studied to establish the role of the glands in animal life. Morphometric parameters reflecting the functional state of the organ were measured on histological specimens (10 from each animal): diameters of terminal sections of the glands and their ducts, the height of the secretory epithelium cells, and volumes of their nuclei (the number of measurements of each parameter was 100, 50, 100, and 200, correspondingly). It is established that the preorbital gland in the red deer is a complex of sebaceous and sweat glands and performs a marking function, as evidenced by the increase in its activity in the summer period. Meibomian glands are modified sebaceous glands, performing a protective function of the eyes from the impact of various factors that increase their activity in the summer period under the influence of the temperature factor. The caudal gland is a complex structure in males in the studied periods, which does not show the seasonal dynamics of its functional state.
Keywords
red deer, cutaneous gland, preorbital gland, meibomian gland, caudal gland, morphological features, functional state.
Introduction
Skin glands of vertebrate animals are diverse and perform several essential functions (Sokolov, Chernova, 2001). However, they are similarly differentiated, distinguishing two main categories - ordinary and specific glands. Specific skin glands are present in all mammalian classes and are a modification of a complex of normal sebaceous and sweat glands (Sokolov, 2002). Many skin glands have a marking function and are peculiar "scent organs", as well as serve for such processes as identification of individuals of their species, attraction of individuals of the opposite sex ("gonads"), and defense (scent repelling) (Shabadash, Chernova, 2006).
The morphology of skin glands, including deer glands, is species-specific. They may differ even within the same species at the subspecies level. This is primarily due to the evolution of each species, its behavior, and habitat (Rzhanitsyna and Ovcharenko, 1988; Sokolov, 2001; Ovcharenko et al. 2016; Ovcharenko et al., 2018; Ovcharenko, 2019; Ovcharenko, 2020). Cutaneous glands are characterized by extreme diversity in their localization, extent, and structure (Motagna, 1962; Motagna, Parakkal, 1974; Sokolov, 1973; Stepanova, 2001). It is known that the degree of skin gland development depends on the sex of the animals, their age, reproductive state, and season of the year. As a rule, they are better developed in sexually mature animals compared to young ones. At the same time, many mammals function more intensively during the breeding period than during the rest of the time. Many authors point out that certain skin glands undergo a reduction when animals are castrated, and at the same time, their development is intensified when sex hormones are injected (Sokolov and Chernova, 2001). The red deer, one of the subspecies of red deer (Cervus elaphus sibiricus, Severtzov, 1872), as a representative of ungulate animals, is a unique object that lives in the wild in the Altai mountains and is bred in red deer farms to obtain valuable products such as antlers, blood, tails, penises, and embryos (Lunitsyn, 2004). Detailed research of skin glands of red deer, including specific glands, is carried out by employees of the Zoology and Physiology Department of Altai State University, in particular, professor Ovcharenko N.D. and her pupils last some years. Their varieties in red deer, among others, are preorbital, caudal, and limb glands. There are single fragmentary data on the general morphology of the preorbital and meibomian glands in the red deer in the studied literature.
This study aimed to investigate the morphological features and changes in morphometric parameters of the preorbital, meibomian, and caudal glands of red deer (Cervus elaphus sibiricus, S., 1872) in different seasons of the year.
Materials and Methods
The object of the study was male red deer of mature age (7-9 years) with a number of 20 animals. The material for the study was the preorbital, meibomian, and caudal glands of the animals obtained during their planned slaughter. The collection was made in winter and summer periods of the year in the farm "Novotalitskoe", Charyshsky District of Altai Krai (51°24′N, 83°34′E). For the research of the seasonal dynamics of a structural and functional condition of glands of animals, the selection of material was made in different periods of the year taking into account features of the biology of red deer: in winter (period of relative sexual rest) and the middle of summer - the period of cutting of antlers (nonossified horns).
As a fixative for the material, a 10% solution of neutral formalin was used. The material was poured into paraffin using an automatic histological guiding system TRS 15 duo to make histological blocks. Paraffin sections were obtained using an HM 325 Thermo rotary microtome. The preparations were stained with hematoxylin-eosin and Van-Gizon (Ovcharenko et al., 2013). Morphometric examination of the glands was performed using Scope Photo 3.0 software. Microphotos were taken using a LOMO Mikmed 6 microscope and a ScopeTek DMC310 camera.
Such parameters as the diameters of the secretory end sections and their ducts, height of the secretory cells, and the diameter of their nuclei were used to evaluate the functional activity of the glands. At least 50 measurements were taken for each parameter (100, 50, 100, and 200, respectively). Ten histological preparations were examined for each animal.
Statistical processing of the obtained data was performed using the Statistica 10 software.
Results
The preorbital gland is a skin fold with strongly thickened walls forming a cavity in which secretion accumulates. Sebaceous and apocrine sweat glands represent the secretory apparatus of the gland. Sebaceous glands have a relatively uniform distribution, and sweat glands are found only in the area of the thickened upper fold of the preorbital sac. The depth of sweat glands and the diameter of their terminal parts change with the season.
Externally, the preorbital gland is a skin fold with significantly thickened walls forming a cavity. The appearance of the preorbital gland is shown in Fig. 1.
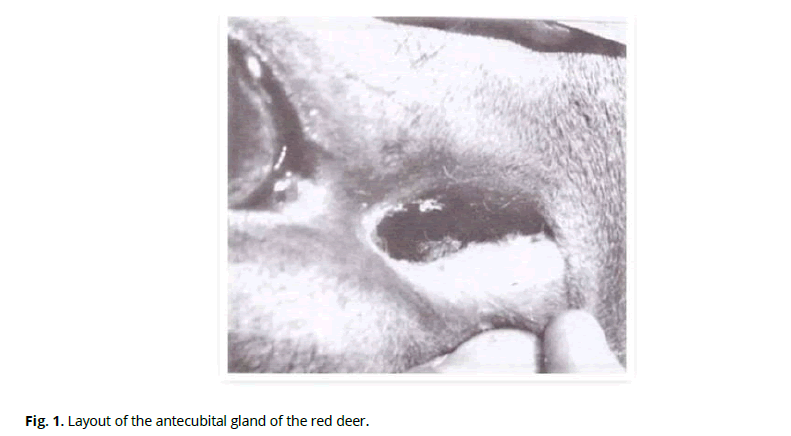
Figure 1: Layout of the antecubital gland of the red deer.
Accumulation of the secretion of the gland occurs in the cavity and is a viscous yellow-brown substance. The cavity has the form of a slit with convex dorsal and concave ventral walls on the cross-section. An S-shaped preorbital slit, which connects to the inner angle of the eye with a shallow sulcus, leads to the cavity of the fold. We found that the sulcus length in adult female red deer is, on average, 15±2 mm, and the slit of the pouch is 28±2 mm. The thickness of the dorsal skin fold is 11.0±0.9 mm, and the ventral one is 9.0±0.7 mm. The most significant thickness, 13.0±1.5 mm, of the fold is at the mouth of the sac, the so-called nasal part of the gland. The most negligible thickness of the fold is in the place of its transition to the sulcus - 4.0±0.5 cm (aboral pole).
The total thickness of the skin of the preorbital sac is 1.7±0.2 mm. Skin epidermis includes basal, thorny, and horny layers. The glossy and granular layers are absent. Derma is well developed, with two distinct layers - papillary and reticulate. A powerfully developed muscular layer underlies the skin. Sparse guard hairs and downy hairs are found. The hair follicle is provided with packets of sebaceous glands. Ducts of sweat apocrine glands are located at greater depth (Fig. 2).
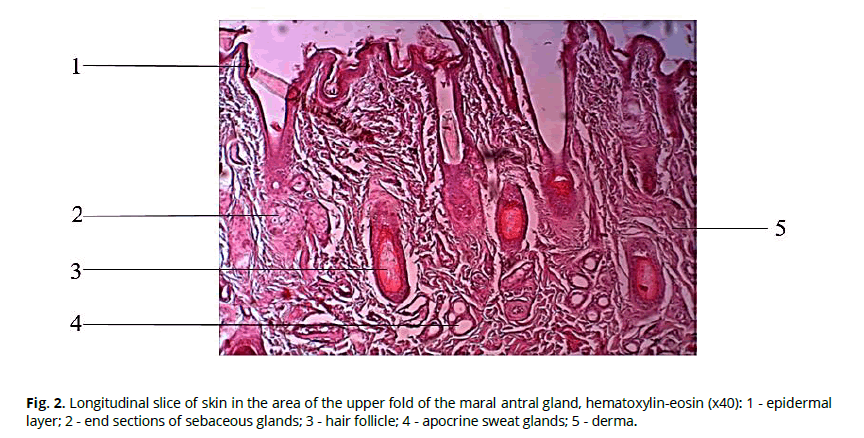
Figure 2: Longitudinal slice of skin in the area of the upper fold of the maral antral gland, hematoxylin-eosin (x40): 1 - epidermal layer; 2 - end sections of sebaceous glands; 3 - hair follicle; 4 - apocrine sweat glands; 5 - derma.
The sweat glands in this area belong to the multicellular monoptichial glands with the apocrine type of secretion. They have a typical tubular structure and are located in the lower layers of the papillary layer. The secretory part forms a ball, which then meanders into a long exit duct (Fig. 3). Sweat glands were found only in the skin of the upper fold of the sac. Sweat glands were absent in other areas. The depth of the sweat glands averaged 647±76 µm.
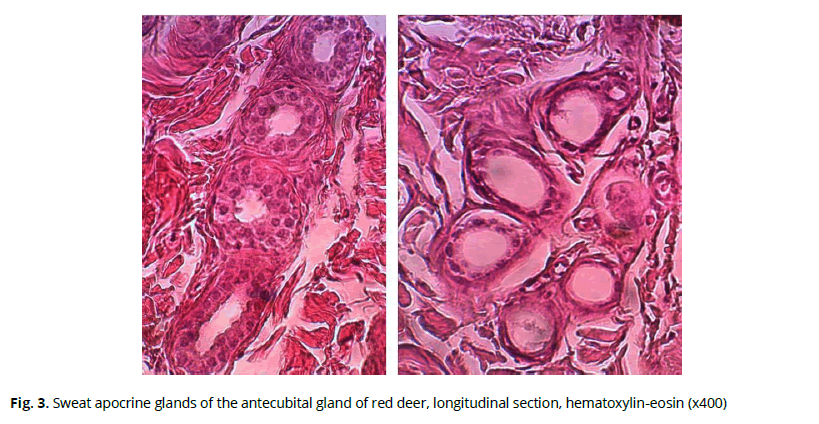
Figure 3: Sweat apocrine glands of the antecubital gland of red deer, longitudinal section, hematoxylin-eosin (x400)
The skin in the sulcus area is characterized by a smaller thickness of the dermal layer compared to the pouch area. The epidermis is also well developed, with distinguishable basal, spiny, and horny layers. Sebaceous glands accompany rare hairs. Apocrine sweat glands are absent. Sebaceous glands are multidollicular and are located one or two in each hair follicle (Fig. 4).
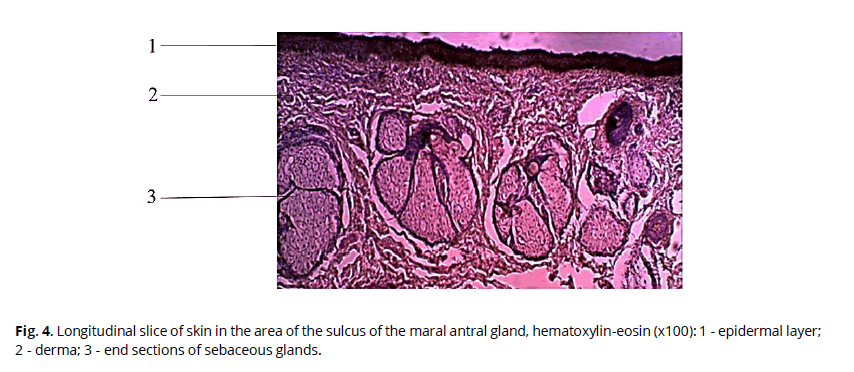
Figure 4: Longitudinal slice of skin in the area of the sulcus of the maral antral gland, hematoxylin-eosin (x100): 1 - epidermal layer; 2 - derma; 3 - end sections of sebaceous glands.
They belong to multicellular polyptychial glands with the holocrine type of secretion (Chernova, 2008). The depth of the terminal sebaceous glands in the area of the pouch and sulcus varies from 426±54 to 611±68 microns. The maximum size of the end sections of sebaceous glands reaches the area of the sac's mouth. The sizes of the end sections of the sebaceous glands in the area of the sulcus and pouch averaged 148±52 × 165±47 μm.
There was an increase in the size of the end sections of sebaceous glands in the investigated individuals in the summer period (292±42 × 307±35 microns), in comparison with the winter period (136±60 × 118±62 microns). An increase in the diameter of the channel of sweat apocrine glands in the studied area (85±4 µm) compared to the winter period (56±9) is also noted in summer.
It is also known that meibomian glands are free; they are not connected with hair (Gutgesell et al., 1982). We investigated the area of the upper eyelid of male red deer. The glands are represented by clusters of secretory acini - alveoli, which surround the central canal, connected to it by ducts. One end of the duct is blind, while the other opens close to the skin surface, covered with a mucous membrane, where the secret of the glands is secreted. A cross-section of the meibomian gland of the upper eyelid of a male red deer is shown in Fig. 5.
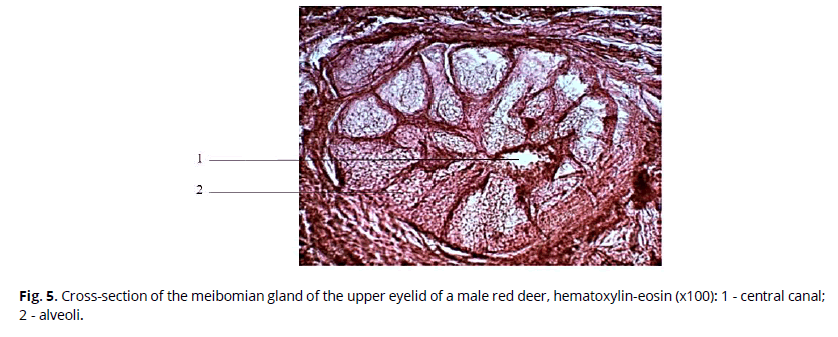
Figure 5: Cross-section of the meibomian gland of the upper eyelid of a male red deer, hematoxylin-eosin (x100): 1 - central canal; 2 - alveoli.
We found that the transverse size of the alveoli can reach 352 µm. Alveoli are connected to the central canal by small ducts. On average, 23 alveoli surround the canal. The central canal has a broader lumen, ranging from 177 to 229 μm in diameter. It is known that the system of ducts contributes to the additional transformation of the secretion due to the microorganisms living there, which secrete several enzymes. Thus, there is a breakdown of triglycerides into free fatty acids and the formation of small portions of mono- and diglycerides (Knop et al., 2011).
Comparison of the morphometric indices of the meibomian glands of the upper eyelid in winter and summer (Fig. 6) suggests seasonal differences in such a parameter as the diameter of the central canal. It is considerably more significant in the summer period than the winter one, while the transverse size of the alveoli changes insignificantly during the year.

Figure 6: Morphometric index of the meibomian glands of the upper eyelid (μm, M±S)
The caudal gland of the maral is one of its most significant glandular structures. Functionally, the caudal gland belongs to the group of communicative signal glands. The primary purpose of this gland is, presumably, warning of danger - the release of "alarm odor" (Sokolov and Chernova, 2001).
From the morphological point of view, this gland is a dark-colored subcutaneous gland that surrounds the caudal vertebra in the form of two bands. The thickness and length of the glandular field differ in different parts of the tail (Fig. 7).

Figure 7: Topography of the caudal gland of red deer (male, seven years old)
Skin in the area of the caudal gland of red deer has a typical structure. The total thickness of the epidermis on average in the tail area is 110-150 microns, with an expressed predominance of stratum corneum (68.04+11.95 microns). The dermis forms a strong wavy knot, with longitudinal horizontal collagen bundles between the follicles. The root layer is densely saturated with hair follicles. Hair in this area occurs in three types: pinnate, covering, and down. The bulbs of the hair follicles lie at the level of the upper border of the glandular field. The average depth of the gland, counting from the epidermis, is 1000-1400 microns.
On histological sections, the glandular tissue retains a lobular structure, between which there are elements of stroma - interlayers of connective tissue, in which interlobular discharge ducts, blood vessels, and elements of the musculature lie. The thickness of interlayers varies in different sections. The lobular structure of the gland is particularly well expressed on cross-sectional preparations, where the average size of the lobes is 999.36 × 607.02 microns (Fig. 8). Volumetric ratios of the stroma and parenchyma of the gland per unit area are shifted in favor of the secretory part.
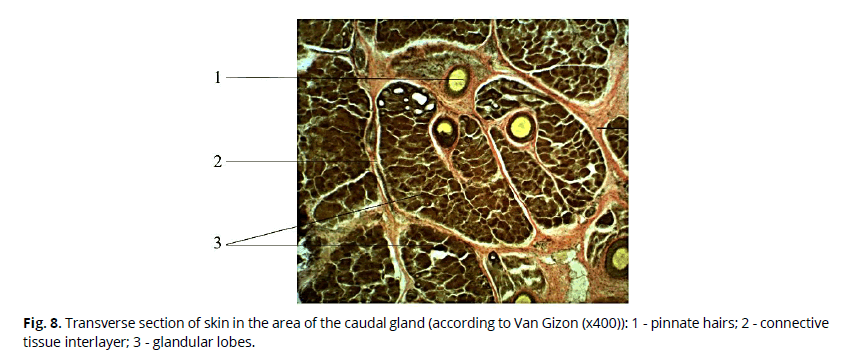
Figure 8: Transverse section of skin in the area of the caudal gland (according to Van Gizon (x400)): 1 - pinnate hairs; 2 - connective tissue interlayer; 3 - glandular lobes.
The glandular apparatus of the skin and its derivatives in the caudal region, is represented by sebaceous and modified sweat apocrine glands, the latter predominating in volume and mass (Fig. 9).

Figure 9: Longitudinal section of skin and caudal gland of red deer (according to Van Gizon (x400)): 1, epidermis; 2, dermis; 3, sebaceous glands; 4, merocrine sweat glands; 5, apocrine sweat glands.
The uppermost location in the studied organ is the sebaceous glands, which are directly connected with hair follicles (following along the hair). The depth of sebaceous glands is from 264 to 956 microns. Depending on the size and number of terminal lobules, they can be divided into the first, second, and third orders. The largest - of the first order have up to 5-6 lobes and are connected through withdrawal ducts (width 32-55 microns) with pin hairs located deeper than the others. Alveoli with a predominance of differentiating (maturing) forms of sebocytes, with an average diameter of 17.78-18.20 μm, are found more frequently. The nuclei of these cells have a slightly oval shape, and their diameters are 6.82-7.04 μm. The cytoplasm contains lipid droplets of different sizes (average diameter 2.83+0.43 μm). On the basal membrane of each sebaceous acini, there are undifferentiated spindle-shaped cells (cambial sebocytes) with large nuclei. The inner part of the secretory section is occupied by more giant cells of rounded or polygonal shape (secretory sebocytes) with a very light cytoplasm and a round oval nucleus. The cells are tightly confined to each other and fill the entire space inside the acinus. The follicles of the hair follicles have well-developed muscles, which run at an angle to the skin surface, providing hair lifting and secretion of glandular secretions.
In the lower layer of the dermis, and sometimes a little lower, there are common merocrine sweat glands with a tubular structure in the upper part of the hypodermis.
The central part of the glandular mass is represented by the modified apocrine sweat glands, representing a vast formation. The size and shape of the lobes are varied. Smaller lobes (350-370 µm in diameter) are rounded or flattened in shape and are located in the upper layers. Large lobes have a polygonal shape, with an average diameter of 1320 µm. They are oriented mainly perpendicular to the skin surface. The terminal parts of the lobes have a complex polyalveolar structure. The alveoli (average diameter 53.99+4.66 μm) consist of one row of cylindrical cells, with clearly visible intraacinar ducts (diameter 16.75+4.01 μm), filled, in some places, with an amorphous structure and isolated by large rounded cells with a small nucleus. Many authors consider these formations as a secretion. The number of cells composing the terminal compartment is from 8 to 20. The cytoplasm of secretory cells has clearly visible granularity and oxyphilic staining. The nuclei are rounded, and most cells are shifted to the apical part. The height of the secretory epithelium averages 21-22 µm. In the upper part of the glandular parenchyma, there are alveoli with dilated secretory tubes up to 212 μm, and their walls are represented by low cubic epithelium, 12-14 μm high. The alveoli are surrounded by a layer of myoepithelial cells lying on the basal membrane (Fig. 10).

Figure 10: Alveoli of the maral tail gland (according to Van Gizon (x400)): 1 - myoepithelial cells; 2 - cells of the secretory epithelium; 3 - nuclei; 4 - secretion.
Analyzing the indices of alveolar diameter, cell height, and nucleus diameter in adult males depending on the year's season, we found that only the diameter of acinar cell nuclei differed significantly (Table 1). The difference between the other indices was not statistically significant.
Table 1. Morphometric indices of the caudal gland of adult males depending on the season
| Season | Alveol diameter (d, μm) |
Cell height (h, μm) |
Nuclei diameter (d, μm) |
|---|---|---|---|
| winter | 67.00±1.91 | 18.36±0.62 | 4.95±0.07 |
| summer | 67.23±1.02 | 19.26±0.3 | 5.80±0.07 |
This testifies that at the beginning of summer compared to winter, the activity of the caudal gland in males is still practically unchanged. We assume that the processes of activation of its functional state, which is evidenced by the increase in the volume of nuclei of secretory cells, will reach the maximum value by the end of summer when the preparation of males for rut begins and "odor signaling" will be necessary for animals. Besides, in the semi-free keeping of red deer, during the growth and cutting of antlers, the animals are in separate parks, isolated from females and young animals with which they are united at the end of summer.
Conclusions
Our results partially agree with available literature data about the seasonal variability of skin cover in mammals. In most animals, normal sebaceous glands remain the same in winter and summer, while sweat glands are subject to seasonal dynamics (Sokolov, 2002). Considering that the preorbital gland in red deer is a complex of sebaceous and sweat glands and has a marking function, an increase in its activity in summer is objective and expedient. Meibomian glands are modified sebaceous glands, performing a protective function of eyes from various factors that increase their activity in the summer period under the influence of the temperature factor. The tail gland being a complex structure in males in the studied periods, does not demonstrate the seasonal dynamics of its functional state.
References
Chernova O.F. (2008). Glands of external covers of chordates in the aspect of morphobiological theory of evolution by A.N. Severtsov. Zoological Studies: Proceedings of the Zoological Museum of Moscow State University. Мoscow.
Gutgesell, V.J., Stern, G.A., Hood C.I. (1982). Histopathology of meibomian gland dysfunction. J. Ophthalmol, 94, 389–392.
Knop, E., Knop, N., Millar, T., Obata, H., Sullivan, D.A. (2011). The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci, 52(4), 1938–1978.
Lunitsyn, V.G. (2004). Antler reindeer breeding in Russia. Siberian Branch of the All-Russian Research Institute of Reindeer Husbandry. Barnaul.
Montagna, W. (1962). The structure and function of skin. Second edition. New-York-London: Academic Press.
Montagna, W., Parakkal, P. F. (1974). Structure and Function of Skin. Academic Press: New York.
Ovcharenko, N.D. (1988). Species, age, and seasonal features of hystomorphology and innervation of the skin of spotted deer. Thesis of Doctoral Dissertation. Barnaul.
Ovcharenko, N.D., Kuchina E.A., Teterina, A.V. (2011). Features of macro- and microstructure of the tail gland of red deer (Cervus elaphus sibiricus, Severzov, 1872) depending on sex. Actual problems of agriculture of mountain territories. Proceed. III Int. Sc. Conf. Gorno-Altaisk, Publishing house.
Ovcharenko, N.D., Kuchina, E.A. (2018). Influence of environmental factors on the structural and functional state of its specific skin gland of Red deer (Cervus elaphus sidiricus Severtzov, 1872). Ukrainian Journal of Ecology, 8(4), 469-475.
Ovcharenko, N.D., Kuchina, E.A. (2019). Morphology of marking glands of red deer (Cervus elaphus sibiricus, Severzov, 1872). Morphology, 155(2), 220.
Ovcharenko, N.D., Kuchina, E.A., Chertovskikih, E.E. (2020). Structural and functional state of the caudal gland in the red deer (Cervus elaphus sidiricus Severtzov, 1872) during different periods of physiological state. Morphology, 157(2-3), 157-158.
Ovcharenko, N.D., Kuchina, E.A., Gib, A.V. (2016). Comparative characteristics of the hystostructure of skin glands in red deer (Cervus elaphus sibiricus, Severzov, 1872) in the eyelid and preocular glands depending on the season. Biodiversity, ecological problems of the Altai Mountains and adjacent regions: present, past, and future. Proceed. IV Int. Conf. Gorno-Altaisk. Publishing house Gorno-Altaisk State University.
Ovcharenko, N.D., Kuchina, E.A., Tuzikova, R.V. (2013). Histological and histochemical methods of research. Barnaul: Publishing house of Alta State University.
Rzhanitsina, I.S., Ovcharenko, N.D. (1986). Species features of skin and its derivatives in antler deer. Environmental aspects of functional morphology in animal husbandry. Moscow: Nauka.
Shabadash S.A., Chernova O.F. (2006). Hepatoid skin glands of mammals. Moscow: Scientific Publishing House TMK.
Sokolov, V.E. (1973). Mammalian skin. Moscow: Nauka.
Sokolov, V.E. (2002). Selected papers. Volume 1. Morphology, systematics, faunistics, and evolution of mammals. Moscow. Nauka.
Sokolov, V.E., Chernova O.F. (2001). Skin glands of mammals. Moscow. Geos.
Stepanova L.V. (2001). Dermal glands of mammals in historical aspect. Problems of evolutional and ecological morphology. Мoscow.
Author Info
N.D. Ovcharenko*, E.A. Kuchina and A.V. MatsyuraCitation: Ovcharenko, N.D., Kuchina, E.A., Matsyura, A.V. (2021). Comparative morphology of scent glands in marals (Cervus elaphus sibiricus, Severtzov, 1872). Ukrainian Journal of Ecology, 11 (3), 98-105.
Received: 12-Apr-2021 Accepted: 23-May-2021 Published: 31-May-2021, DOI: 10.15421/2021_150
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
