Research - (2022) Volume 12, Issue 5
Cellular investigation on the role of osmotic pressure in limiting the toxic effects of pesticidesin durum wheat (Triticum durum Desf.)
G. Issaad1, H. Berrebbah1, M.R. Djebar1 and R. Rouabhi2*Abstract
Soil salinity is considered a major limiting factor in plant development, given the importance of cereals as an essential nutritional material worldwide. The present study is interested in demonstrating the effects of the salt stress/herbicide interaction on the roots of a plant model: durum wheat (Triticum durum) of the WAHA variety.
The results obtained show that wheat is a plant sensitive to the action of NaCl. However, we have observed morphophysiological and metabolic disturbances in seeds subjected to saline stress in increasing doses, after germination.
The measurement of the enzymatic activity shows a stimulation of the Catalase activity (CAT) accompanied by a production of hydrogen peroxide at the origin of oxidative stress, this increase is proportional to the degree of stress induced. Also, this stress induced the release of electrolytes.
In parallel, we were interested in the toxicity of glyphosate. The penetration of the herbicide inside the plant has led to remarkable morpho-physiological and biochemical disturbances.
Regarding the NaCl/Glyphosate interaction, we note a clear improvement in the majority of the measured parameters, this can be considered as an antagonism effect between the two stresses allowing better root growth.
Keywords
Durum wheat, Glyphosate, Salinity, Oxidative stress, Membrane integrity, Catalase, Synergy, Antagonism.
Introduction
Plants play a major role in the terrestrial ecosystem, an essential constituent of the biosphere; they are the source of the oxygen we breathe, transforming the CO2 in the atmosphere into O2. Soil salinity is a major problem in agriculture that limits plant growth and causes significant loss of crop productivity worldwide (Allakhverdiev, 2000; Munns, 2002). Salinity affects up to 20% and 50% of the total cultivated and irrigated land in the world, respectively (Cheng, et al., 2012). However, the use of saline water in agriculture is gradually increasing owing to shortage of fresh water.
In the face of stress, plants develop adaptation strategies to respond to environmental changes by controlling and adjusting their metabolic systems. However, this is not without consequences leading to the appearance of oxidative stress characterized by the formation of Reactive Oxygen Species (ROS). The uptake of pesticides by the roots varies between plants and chemicals and can be greatly influenced by adjuvants and environmental conditions. (Wanga and Liu, 2007).
Materials and Methods
Conduct of tests
The biological material used in this work is durum wheat from the Poaceae family more precisely (Triticum durum Desf). The organs chosen to carry out this study are the roots. The samples come from Sedrata (Souk Ahras), Algeria. We used the WAHA variety. The herbicide used is Glyphosate.
Durum wheat seeds are rinsed with a solution of distilled water, then soak for 24 hours. The seeds are meticulously chosen before their use (No breaks or apparent signs of abnormalities).
To assess the effect of the interaction between salinity and herbicide on the durum wheat variety, we prepared watering solutions with the following concentrations: C1:0 (control) C2:20 µM, C3:50 µM, C4:100 µM, C5:200 µM) of NaCl and a constant concentration of Glyphosate: C:20 µmol. For each dose of NaCl, Glyphosate and each interaction, five repetitions were performed. The treatment solutions are added through daily watering at the rate of 8 ml per Petri dish, during the germination phase (4 days), 40 ml of NaCl, 40 ml of glyphosate, 20 ml of NaCl and 20 ml of the herbicide (glyphosate) for those of interaction, after sowing seeds in pots containing potting soil.
Studied parameters
The germination percentage: The percentage of germination is identified after 48, 72 and 96 hours of treatment. The number of seeds which have sprouted and whose length of at least one of their roots exceeds 2 mm is considered having sprouted (Ben Hamouda, et al., 2001).
Relative water content: The relative water content is an indicator of the hydric state of the plant. The relative water turgor is determined by the percentage of water present in the excised roots after treatment. The calculation is made according to, Ladigues (1975), according to the following formula:
RWC=(FW-DW)/(TW-DW) × 100
RWC: Relative water content.
FW: Fresh weight
DW: Dry weight
TW: Turgid weight
Membrane integrity: The percentage of cellular integrity consists of measuring the release of electrolytes generated by the partial destruction of the plasma membrane.
In this method we first took of 3 samples (stage 1 slip) from each level of stress, then rinsed them with distilled water, and then cut them into small pieces of 1 cm and collected into test tubes containing 10 ml of distilled water.
The tubes are tightly closed with cotton and aluminum foil and maintained 24 hours at room temperature.
After 24 hours, the first interpretation is performed using a conductivity meter while gently placing the probe in the tube after calibration of the device.
The second reading is performed after 24 hours of the first time after tubes autoclaving at 80°C for 20 minutes.
We take 0.5 g of plant material (roots) which are placed in 20ml of distilled water for 48h at 25°C. They are left to cool. Then we add 0.5 ml of H2SO4 and we let them in ice for 1 h. The samples reading are carried out using a spectrometer at a wavelength of 365 nm (Grieve and Grattan, 1983).
Enzymatic determination
Preparation of the enzymatic extract: The enzymatic extract used in our work is prepared for the determination of catalase activity.
After 14 days of treatment, the fresh roots of both varieties of T. durum (1 g) are ground cold with a mortar in 5 ml of phosphate buffer (50 mM phosphate, pH 7.5). The homogenate is then filtered with an adequate cloth before cold centrifugation (12000 g for 20 min). The supernatant obtained is used as an extract determine the enzymatic activity.
The method used is that of (Cakmak and Horst, 1991, Boscoloa etal., 2003). The decreasing in absorbance is recorded withing three minutes for a wavelength of 240 nm and a molar linear extinction coefficient ε=39400 M-1.cm-1.L
Reaching a final volume of 3 ml, the reaction mixture contains: 100 μl of the crude enzyme extract, 50 μl of 0.3% H2O2 hydrogen peroxide and 2850 μl of phosphate buffer (50 mM, pH 7.2).
The calibration of the device is done in the absence of the enzyme extract. The reaction is triggered by the addition of hydrogen peroxide. The catalase activity is expressed in nmol/min/mg of proteins.
Determination of hydrogen peroxide: Grind 0.1 g of roots and 5 ml of 1% TCA Trichloroacetic Acid in an ice bath. The homogenate is centrifuged at 12000 g for 15 minutes.
0.5 ml of the supernatants are mixed with 0.5 ml of regulator (potassium phosphate KOH) and 1 ml of potassium iodine (KI). The D.O is determined at 390 nm using a spectrophotometer (Loreto and Velikova, 2001).
Results
Percentage of germination
The Fig. 1 shows the effect of salt stress on the percentage of germination of the treated seeds.

Fig 1: Effect of salt stress on germination.
Fig. 1 represents the germination percentage of durum wheat seeds of the WAHA variety measured during 4 days (48 h, 72 h and 96 h). Treatment with NaCl shows that the percentage of germination increases with time and decreases compared to the control. The statistical study reveals a significant decrease in the percentage of germination of seeds treated with NaCl, at 24 h and 72 h. This drop reached 70% at the highest concentration.
Relative water content (RWC)
The Fig. 2 represents the effect of salt stress on the relative water contents (RWC) of the roots.
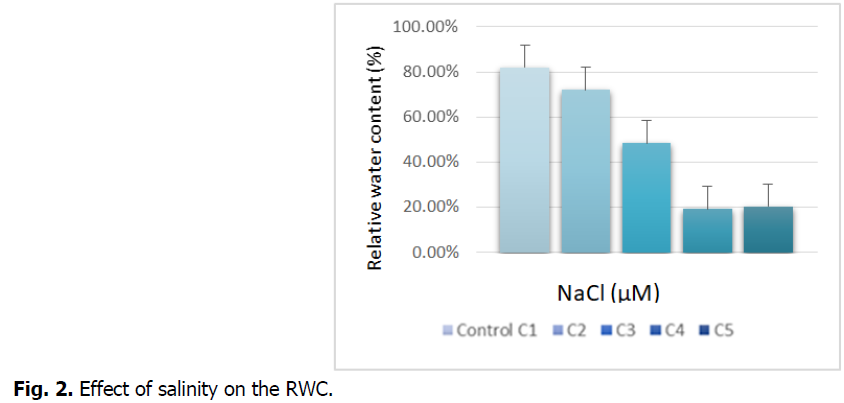
Fig 2: Effect of salinity on the RWC.
We see in the figure above that the treatment with NaCl tends to reduce the relative water content compared to the control. The treatment at the highest concentrations (C4 and C5) records a significant decrease, which is around 75%.
Membrane integrity
The figure shows the effect of salt stress on the integrity of the root membrane.
Fig. 3 shows the integrity of the membrane, expressed as the percentage of electrolytes released at the roots of durum wheat treated with increasing concentrations of NaCl. The figure records an increase in the release of electrolytes in the roots exposed to the concentrations: C3 and C4. Treatment with the highest concentration (C5) leads to a decrease in the leakage of electrolytes compared to the control. This decrease is around 01%.
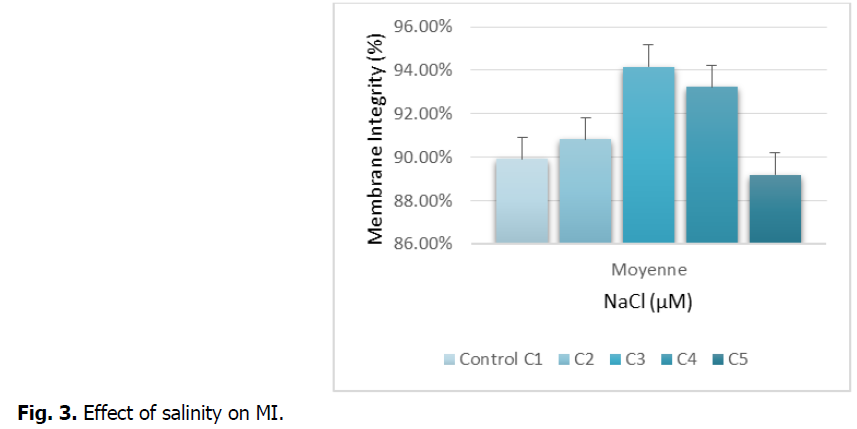
Fig 3: Effect of salinity on MI.
Catalase activity (CAT)
The Fig. 4 below shows the evolution of catalase activity in the roots of durum wheat treated with the different concentrations of NaCl.
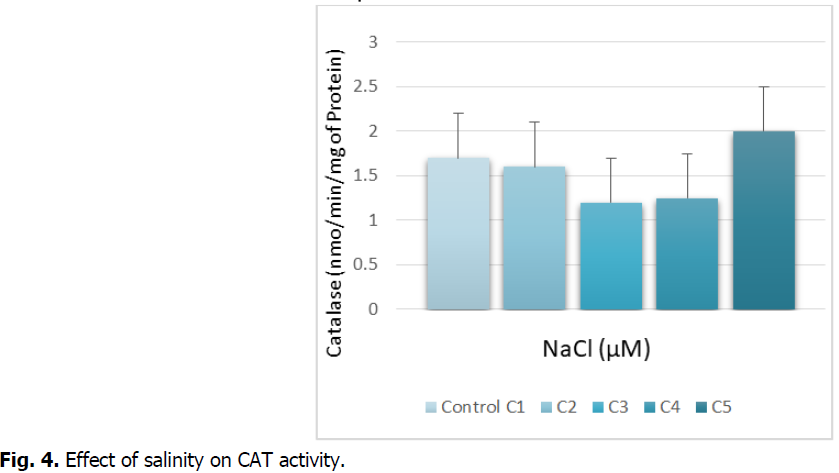
Fig 4: Effect of salinity on CAT activity.
The Fig. 4 indicates the catalase activity is inhibited at the C3 and C4 concentration. This decrease is around 20% compared to the witness. On the other hand, we record a stimulation of the catalase activity at the highest concentration. This increase is around 10%.
Hydrogen peroxide content
The Fig. 5 shows the production of hydrogen peroxide in the roots under salt stress.
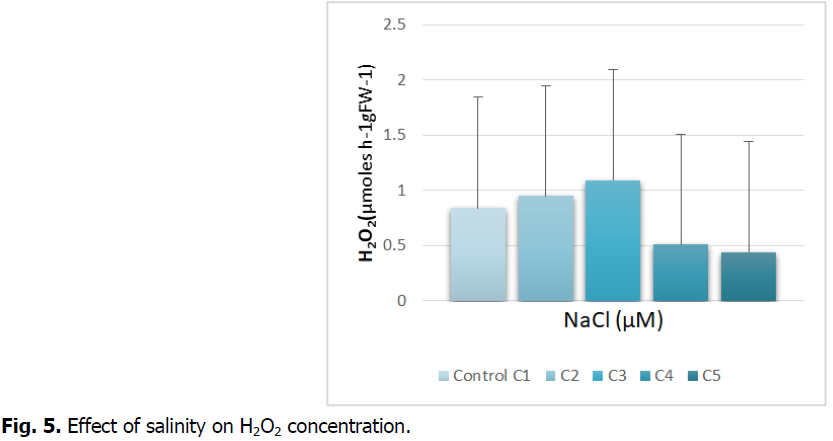
Fig 5: Effect of salinity on H2O2 concentration.
The exposure of the durum wheat roots treated by the different concentrations reveals an increase in the production of H2O2 at the C3 concentration and which tends to decrease at the highest concentrations (C4 and C5).
Effect of glyphosate
Percentage of germination
The Fig. 6 shows the effect of glyphosate on the percentage germination of treated seeds.

Fig 6: Effect of glyphosate on germination.
Fig. 6 shows the germination percentage of durum wheat seeds of the WAHA variety measured for 4 days (48 h, 72 h and 96 h). Treatment with glyphosate shows that the percentage of germination decreases with time and compared to the control. On the other hand, there is an increase at 72 h in the seeds treated with C3.
Relative water content (RWC)
The Fig. 7 shows the effect of Glyphosate on the relative water contents (RWC) of the roots.
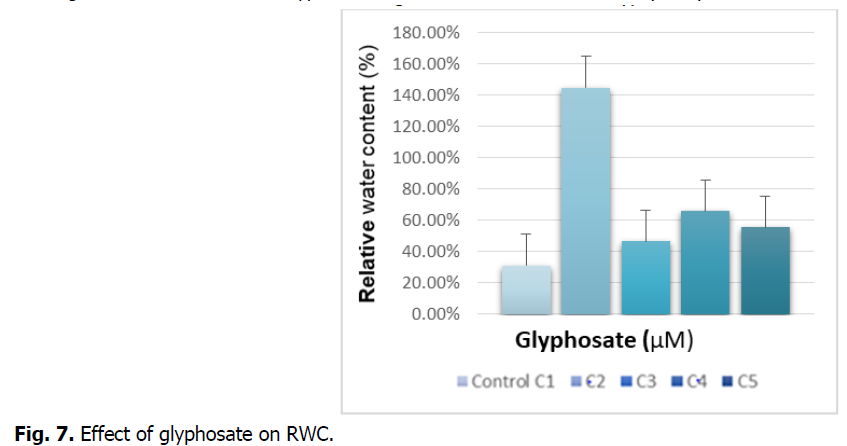
Fig 7: Effect of glyphosate on RWC.
We see in the figure above that treatment with glyphosate tends to increase the relative water content compared to the control. Treatment with concentration (C2) records a significant increase, which is around 300%.
Membrane integrity
The Fig. 8 illustrates the effect of glyphosate on the integrity of the root membrane.
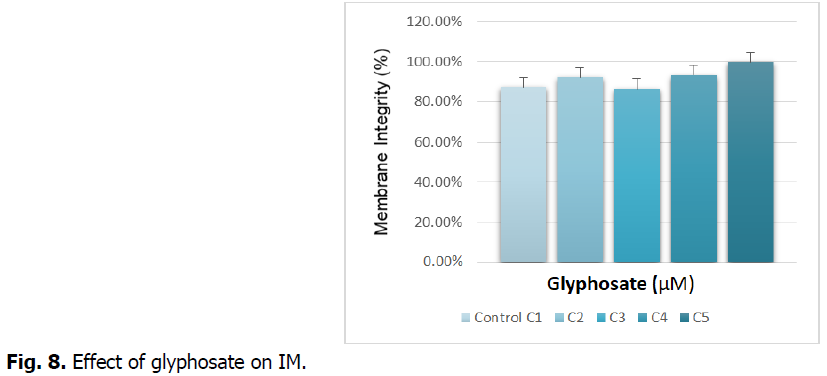
Fig 8: Effect of glyphosate on IM.
Fig. 8 shows the integrity of the membrane expressed as the percentage of electrolytes released at the roots of durum wheat treated with increasing concentrations of glyphosate. The figure records a disturbance in the release of electrolytes in the roots. Treatment with the highest concentration (C5) leads to an increase in the leakage of electrolytes compared to the control. This increase is around 15%.
Catalase activity (CAT)
The figure illustrates the evolution of catalase activity in roots subjected to increasing concentrations of glyphosate.
The Fig. 9 indicates the catalase activity is inhibited at the C3 concentration of glyphosate. This reduction is around 100% compared to the witness. On the other hand, we record a stimulation of the catalase activity at the highest concentration. This increase is around 10%.
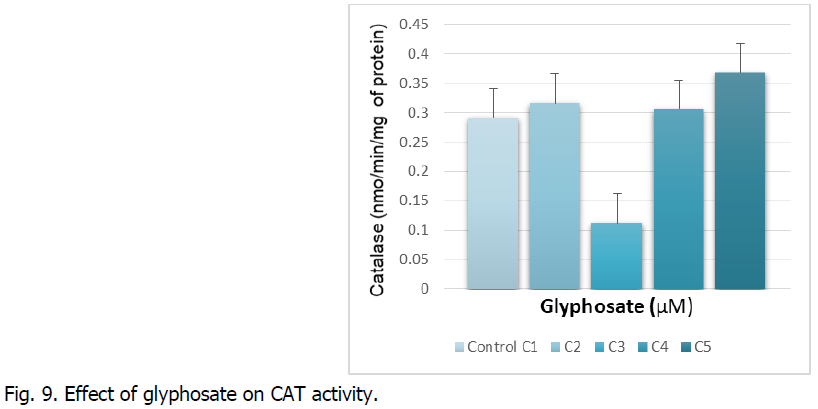
Fig 9: Effect of glyphosate on CAT activity.
Hydrogen peroxide content
The Fig. 10 illustrates the concentration of hydrogen peroxide in roots subjected to increasing doses of glyphosate.
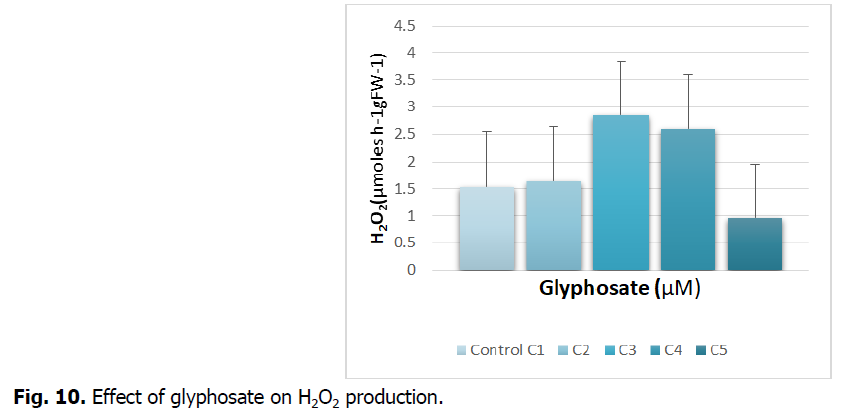
Fig 10: Effect of glyphosate on H2O2 production.
Exposure of durum wheat roots treated with different concentrations of glyphosate reveals an increase in the production of H2O2 at concentrations C3 and C4. This increase is around 90% and tends to decrease at the highest concentration (C5).
Effect of the interaction between salt stress and glyphosate Percentage of germination
The Fig. 11 illustrates the NaCl/glyphosate cocktail effect on the germination percentage of the treated seeds.
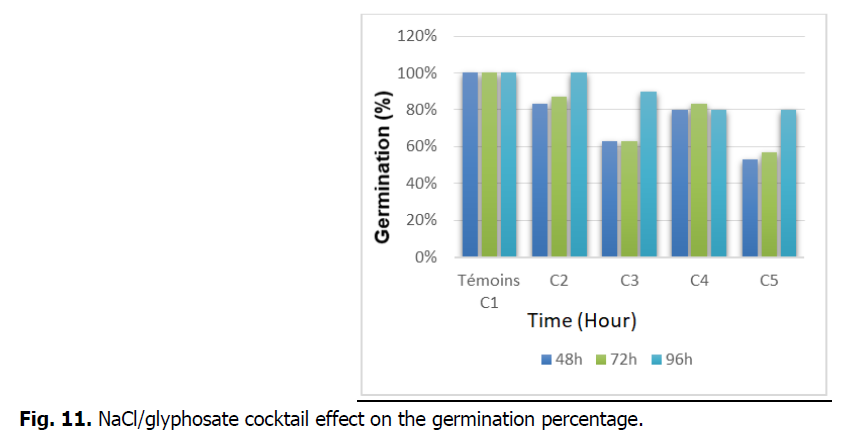
Fig 11: NaCl/glyphosate cocktail effect on the germination percentage.
Fig. 11 represents the germination percentage of durum wheat seeds of the WAHA variety measured during 4 days (48 h, 72 h and 96 h). The cocktail effect shows that the percentage of germination decreases with time and compared to the control. On the other hand, there is no effect recorded at 96 h in seeds treated with C3.
Relative water content (TRE).
The Fig. 12 illustrates the NaCl/glyphosate cocktail effect on the relative water contents (RWC) of the roots.
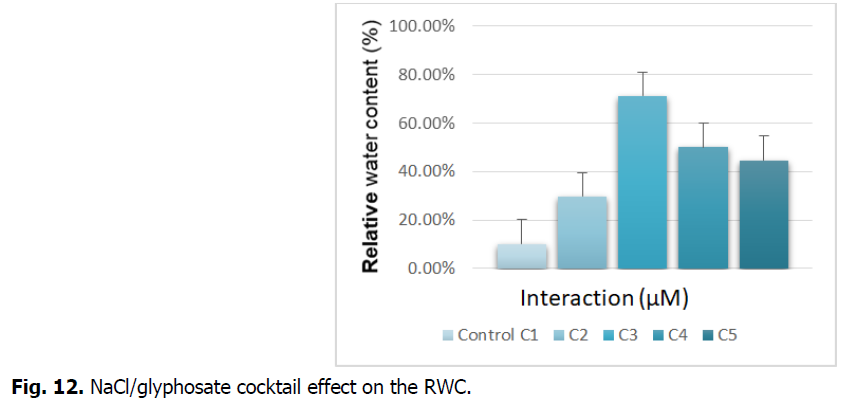
Fig 12: NaCl/glyphosate cocktail effect on the RWC.
We see in the figure above that the treatment with NaCl/glyphosate tends to increase the relative water content compared to the control. Treatment with concentration (C3) records a significant increase, which is around 600%.
Membrane integrity
The Fig. 13 illustrates the NaCl/glyphosate cocktail effect on the integrity of the root membrane.
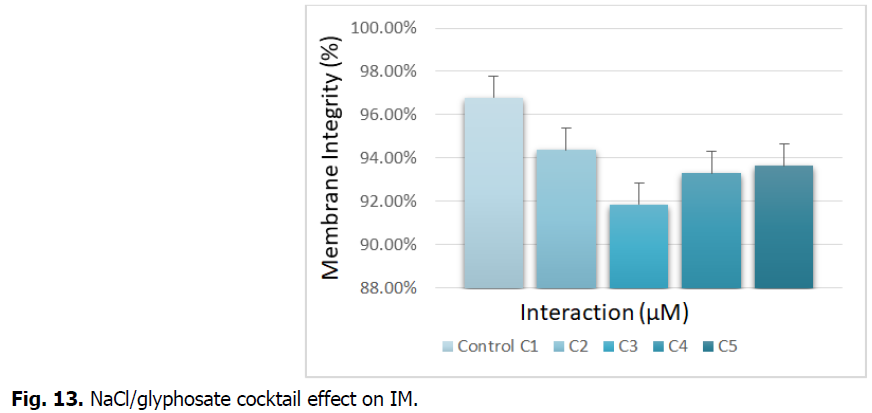
Fig 13: NaCl/glyphosate cocktail effect on IM.
Fig. 13 shows the integrity of the membrane, expressed as the percentage of electrolytes released at the roots of durum wheat treated with increasing concentrations of NaCl/glyphosate. The figure records a decrease in the release of electrolytes in the roots. This recording is important for the concentration (C3), which is around 100% compared to the control.
Catalase activity
The Fig. 14 illustrates the activity of catalase in the roots subjected to the NaCl/glyphosate cocktail effect.
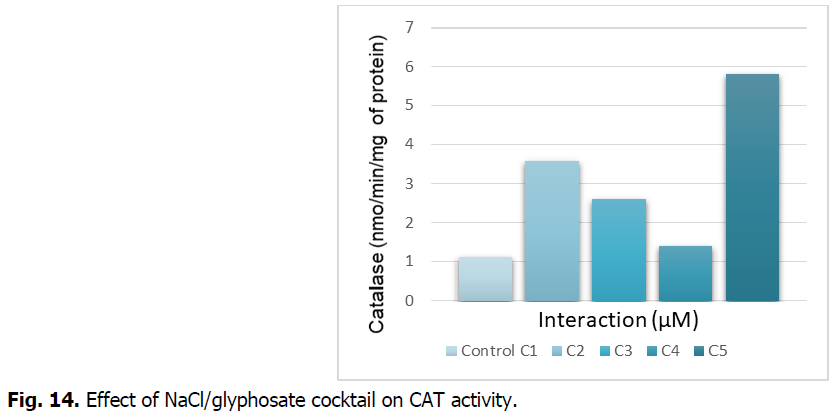
Fig 14: Effect of NaCl/glyphosate cocktail on CAT activity.
The Fig. 14 indicates that the catalase activity is stimulated in the seeds treated with the different concentrations of NaCl/glyphosate. The stimulation is important at the C5 concentration, of the order of 500% compared to the control.
Hydrogen peroxide content
The Fig. 15 shows the evolution of the average hydrogen peroxide content in the roots subjected to the salt stress/glyphosate interaction.
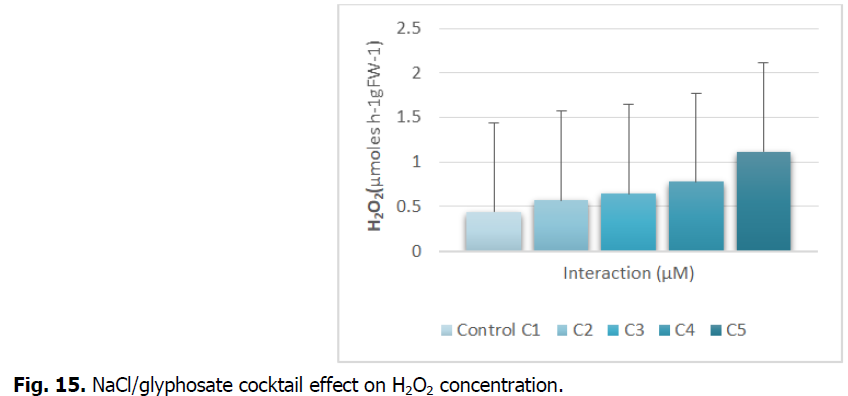
Fig 15: NaCl/glyphosate cocktail effect on H2O2 concentration.
The exposure of durum wheat roots treated by the different concentrations of NaCl/glyphosate reveals an increase in the production of H2O2. This increase is proportional to the stress induced.
Discussion and Conclusion
We will proceed to a general discussion of the results which have just been presented. This discussion will consist of three parts: The first will focus on the effects of salt stress, the second, those of glyphosate, while the third will focus on the interaction of the two molecules.
Salt stress
The study of physiological parameters shows that salinity affects the relative water content and the percentage of germination.
According to our analyzes, the relative water content of the treated seeds has decreased significantly to avoid water loss. According to Hassani, etal., 2008 the absorption of water is maintained at a sufficient level to avoid dehydration of the plant tissues, to establish the phenomenon of succulence and to be able to dilute as many osmolytes as possible. The more the stress increases, the more the relative water content (RWC) drops. This seemingly obvious result is confirmed by numerous studies by Ali Dib, 1992.
The results reported in our study show that wheat is a plant sensitive to the action of NaCl, at the germination stage; the germination of the seeds diminishes when the salt concentration increases, which causes its inhibition. Our results clearly show that the seeds germinate better in the absence of salinity. The same results are reported by Bliss, et al., 1986, which demonstrate that the delay in seed germination is due to the time necessary for the seed to set up mechanisms allowing it to adjust its internal osmotic pressure.
Also the results obtained on the biochemical parameters prove that the salinity has great repercussions on the membrane integrity, the enzymatic activity of catalase and the concentration of hydrogen peroxide.
The integrity of the membrane increases with increasing doses of NaCl. The cell membrane of our plant model is indeed a main site of salt damage causing its dysfunction which leads to an increase in the leakage of ions causing lipid peroxidation, results confirmed by Mansour, 1997, who concluded that l he harmful effect of salinity on the plasma membrane is mainly due to the action of salt ions.
Continuing with the data collected on the CAT assay and the H2O2 concentration, where we observed a great complementarity of the results which reveal a dose-dependent production of hydrogen peroxide. The plants have developed an antioxidant system confirmed by catalase activity which plays an important role in the elimination of H2O2 and this in order to restore cellular homeostasis impaired by salinity (Saraf, 2013; Rouabhi et al., 2006; Henine et al., 2016).
Glyphosate
Herbicides are frequently and abruptly used in intensive cropping systems to optimize agricultural production following accumulation in the soil and subsequent uptake by plants. Herbicides are toxic to many crops, including grains. Among cereals, wheat is considered important because of its high protein nutritional value. (Mohammed and Mohd, 2018)
In our experience, the penetration of glyphosate inside the plant has induced morpho-physiological and biochemical disturbances.
According to the study by Zablotowicz and Reddy, 2007, herbicides considerably reduced the nitrogenase activity of rhizobium (an enzyme complex allowing the symbiosis of rhizobium bacteria with plants through the formation of nodules). Under the effect of increasing concentration of glyphosate the symbiotic events are reached (the formation of nodules; true organs of metabolic exchange). As a result, the symbiotic events decrease, leading to a decrease in the formation of nodules responsible for the metabolic exchange between bacteria and plants.
According to Satchivi, et al., 2000, glyphosate enters tissue and reaches active metabolic sites, such as the plant's meristems, after being translocated through vascular tissue. Similarly, the herbicide can also be transferred to various plant tissues (Ready, et al., 2004). As a result, plant organs exhibit high rates of metabolism and growth which represent important sinks for glyphosate (Hetherington, et al., 1999).
The results relating to the different percentages of the germination of treated seeds show a significant decrease observed following the treatments with increasing concentrations of glyphosate after 96 hours. Thus, some authors link the number of seeds germinated to the rate of proteins synthesized, which suggests that oxidative damage to the roots may be responsible for the observed germination delay (Berova, et al., 2002; Willekens, et al., 1997). However, at low doses the herbicide weakly affects this parameter.
Regarding the relative water content, our results demonstrated that at low dose (C2) glyphosate has a stimulating effect on the development of the root system leading to a very high relative water content compared to controls, or the doses superiors used have a depressive effect on root growth which explains the low water levels observed. According to Boreva and Stoeva., 2000, in the unfavorable conditions of the surrounding environment, the plant is weakened and therefore incapable of making the most of its environment.
The biochemical parameters are also subject to the repercussions of glyphosate. Our results demonstrate that the increase in hydrogen peroxide is accompanied by the increase in membrane integrity, which is the cause of lipid peroxidation, which led to the stimulation of the enzyme trapping ROS: catalase. These parameters are frequently used as indicators of oxidative stress in plants. (Gunes, et al., 2007).
In a gene expression analysis, Ahsan, et al., Found that the application of glyphosate generates hydrogen peroxide (H2O2), resulting in peroxidation and destruction of lipids in Oryzasativa rice leaves.
Glyphosate/salt cocktail effect
The environmental environment of plants is frequently subjected to all types of aggression affecting their growth and development, among these aggressions, biotic (Herbicide) and abiotic (Salt) stress, the flagship subject of our study.
In accordance with the results that we have collected on the plant; the interaction between salinization and glyphosate had a beneficial effect on our treated roots compared to the results obtained for each of the stresses separately (salt and glyphosate).
We suspect that this result is due to an antagonism effect, which means that one of the two stresses applied has block the effect of the other. The salt stress may have been prevented the penetration of the different solutes which explains the reduction in the harmful effects of glyphosate.
Regarding the physiological parameters, we note a clear improvement in the percentage of germination under the effect of the interaction, this can be considered as an antagonism effect between the two stresses allowing better root growth.
The relative water content is slightly affected by this interaction because by reaching the highest doses of combined stress, we notice a regression in the rate of hydration of the roots. This can be explained by the combined action of NaCl and glyphosate which produce an overall toxic effect by synergy.
Concerning the biochemical parameters, our observations always remain in agreement with the results that we obtained later from each of the stresses exerted individually, in fact the increase in hydrogen peroxide is accompanied by the increase in membrane integrity thus disturbing catalase activity. The toxic combination of salt stress/glyphosate works in synergy.
References
Adami, M., Modolo, A., Adami, P. (2017). White clover tolerance to herbicides applied at different rates and phenological stages. African Journal of Agricultural Research, 12:2336-2341.
Ahsan, N., Lee, D.G., Lee, K.W., Alam, I. (2008). Glyphosate-induced oxidative stress in rice leaves revealed by proteomic approach. Plant Physiology and Biochemistry, 46:1062-1070.
Allakhverdiev, S.I., Sakamoto, A., Nishiyama, Y., Inaba, M., Murata, N. (2000). Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiology, 123:1047-1056.
Ali Dib, T., Monneveux, P., Araus, J.l. (1992). Adaptation à la sécheresse et notion d’idéotype chez le blé dur. II. Caractères physiologiques d’adaptation. Agronomie, EDP Sciences, 12:381-393.
Ben-Hamouda, M., Ghorbal, H., Kremar, R.J. (2001). Allelopathic effects of barley extracts on germination an ace ling growth of bread on durum wheats. Agronomie, 21:65-71.
Berova, M., Ziatev, Z., Stoeva, N. (2002). Effect of paclobutrazol on wheat seedlings under low temperature stress. Bulgarian Journal of Plant Physiology, 28:75-84.
Bliss, R.D., Platt-Aloia, K.A., Thomson, W.W. (1986). Osmotic sensibility in relation to salt sensitivity in germinating barley seeds. Plant, Cell and Environment, 9:721-725.
Cheng, Z., Woody, O.Z., McConkey, B.J., Glick, B.R. (2012). Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome. Applied Soil Ecology, 61:255-263.
El-Midaoui, M., Benbella, M., Aït-Houssa, A., Ibriz, M., Talouizte, A. (2007). Contribution À L’étude De Quelques Mécanismes D’adaptation à La Salinité Chez Le Tournesol Cultive (Helianthus annuus L.). Revue HTE, 136:29-34.
Gunes, A., Inal, A., Bagci, E.G. (2007). Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L) rown under B toxicity. Scientia Horticulturae, 113:113-119.
Hassani, A., Dellal, A., Belkhodja, M., Harch, K. (2008). Effet de la Salinite sur L’eau et Certains Osmolytes Chez L’orge (Hordeum Vulgare). European Journal of Seantific Research, 23:61-6.
Henine, S., Rouabhi, R., Gasmi, S., Amrouche, A., Abide, A., Salmi, A. (2016). Oxidative stress status, caspase-3, stromal enzymes and mitochondrial respiration and swelling of Paramecium caudatum in responding to the toxicity of Fe3O4 nanoparticles. Toxicology and Environmental Health Sciences, 8:161-167.
Hetherington, R., Reynolds, T.L., Marshall, G., Kirkwood, R.C. (1999). The absorption, translocation and distribution of the herbicide glyphosate in maize expressing the CP-4 transgene. Journal of Experimental Botany, 50:1567-1576.
Ladiges, P.Y. (1975). Some aspects of tissue water relations in three populations of Eucalyptus viminalis Labill. New Phytologist, 75:53-62.
Loggni, B., Scartazza, A., Brugnoli, E., Navari-Izzo, F. (1999). Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology, 119:1091-1100.
Loreto, F., Velikova, V. (2001). Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology, 127:1781-1787.
Jaiwal, P.K., Singh, R.P., Gulati, A. (1998). Strategies for improving salt tolerance in higher plants. Enfield, N.H. (USA), pp:87-110.
Mohammed, S., Mohd, S.K. (2018). Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia Cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech, 8:131.
Munns, R. (2002). Comparative physiology of salt and water stress, Plant, Cell and Environment, 25:239-250.
Rouabhi, R., Djebar-Berrebah, H., Djebar, M.R. (2006). Toxic effect of a pesticide, diflubenzuron on freshwater microinvertebrate (Tetrahymena pyriformis). Chinese Journal of Applied and Environmental Biology, 12:514-517.
Reddy, K.N., Rimando, A.M., Duke, S.O. (2004). Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. Journal of Agricultural and Food Chemistry, 11:5139-5143.
Satchivi, N.M., Wax, L.M., Stoller, E.W., Briskin, D.P. (2000). Absorption and translocation of glyphosate isopropylamine and trimethylsulfonium salts in Abutilon theophrasti and Setaria faberi. Weed Science, 48:675-679.
Saraf, N. (2013). Enhancement of catalase activity under salt stress in germinating seeds of vigna radiata. Asian Journal of Biomedical and Pharmaceutic Sciences, 3:6-8.
Wang, C.J., Liu, Z.Q. (2007). Foliar uptake of pesticides-present status and future challenge. Pesticide Biochemistry and Physiology. 87:1-8.
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Montagu, M.V., Inzé, D., Camp, W.P. (1997). Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. The EMBO Journal, 16:4806-4816.
Zablotowicz, R.M., Reddy, K.N. (2007). Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. Crop Protection, 26:370-376.
Author Info
G. Issaad1, H. Berrebbah1, M.R. Djebar1 and R. Rouabhi2*2Toxicology and Ecosystems Pathologies Laboratory, Larbi Tebessi university, Tebessa, Algeria
Citation: Issaad, G., Berrebbah, H., Djebar, M.R., Rouabhi, R. (2022). Cellular investigation on the role of osmotic pressure in limiting the toxic effects of pesticidesin durum wheat (Triticum durum Desf.). Ukrainian Journal of Ecology. 12:57-66.
Received: 29-May-2022, Manuscript No. UJE-22-65305; , Pre QC No. P-65305; Editor assigned: 31-May-2022, Pre QC No. P-65305; Reviewed: 11-Jun-2022, QC No. Q-65305; Revised: 17-Jun-2022, Manuscript No. R-65305; Published: 24-Jun-2022, DOI: 10.15421/2022_375
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
