Research Article - (2022) Volume 12, Issue 2
Assessment of water quality of the Cheffia Dam in Algeria based on microbiological quality index and suitability for irrigation
W. Boumaraf1*, A. Bergal1 and A. Delimi1,2Abstract
Cheffia Dam located in El Tarf area has an agricultural vocation, an activity depending on much water to meet the needs of a variety of cultures. Water intended to irrigation must exhibit physicochemical features tolerable by crops. The purpose of this study is to evaluate the quality of the water in Cheffia dam. Three sampling stations were selected over three months: “February, March and April 2019”, to determine the physicochemical and bacteriological parameters of the water. Some physicochemical parameters and heavy metal concentrations were analyzed in water samples (T, pH, EC, DO, Salinity, Turbidity, Cl–, Ca2+, Mg2+, NO2–, NO3–, NH4+, PO43– and Fe2+). The samples taken for the bacteriological study have focused on the research and listing of total germs, faecal coliforms and faecal streptococci. The results indicate that most of the physicochemical parameters in Cheffia dam are within or at the permissible limit to the Algerian standards, and WHO for drinking and irrigation water. The obtained bacteriological results illustrate the presence of significant levels of total coliforms, faecal coliforms and faecal streptococci. The high load of these germs during the study period may be due to the runoff and unhygienic behavior observed in the study area. The calculation of microbiological contamination index confirms the absence of degradation in water quality in which contamination is null. The studied surface waters are of good quality for agriculture and suitable for irrigation of most crops, but attention should be paid to leaching, difficult in marginally permeable soils and to drainage.
Keywords
Algeria, Cheffia dam, Bacteriology, Index, Irrigation, Water quality.
Introduction
The development of agriculture and fertility of soils plain have created dense human settlements in the studied area particularly in North. Research has indicated that agricultural practices may cause nitrate, chlorides and sulfates contamination to be high to exceed the maximum acceptable level for water (Benrabah, 2016). Water is a transversal vector of the ecosystem in interaction with all the elements of the biosphere (Olivaux, 2007; Barbault, 2007) and the development of human activity (domestic, collective, agricultural, industrial). Water is vital for life, but it is also responsible for the death of millions of human beings in the Third World, because of its pollution due to chemicals and microbiological products that make it unfit for consumption (Guiraud, 1998). Today, Algeria has 70 operational dams and 25are in construction. The Cheffia dam, built in 1965, is a dam in normal retaining land of 165 m. It regularizes 95 Hm3 annually for the needs of the irrigated Bounamoussa perimeter (Wilaya El Tarf), Annaba city and regional industries including El Hadjar steel. The water quality had in recent years various problems due to uncontrolled industrial discharges and the intensive use of chemical fertilizers in agriculture. There is a failure in dam waters management especially in distribution. The needs for agricultural water were never satisfied at 100%. Therefore, in the last few years, the control of pollution and water quality became particularly obligatory explodes, in order to preserve the environment and the health of living beings, or to exploit it for human consumption and industrial use (Barbault, 2007). For this reason, this work aims to study the physico-chemical and microbiological quality of water in Cheffia dam, which is characterized by an intense anthropic influence manifested by agricultural activities practiced throughout the catchment area, and knowing that suitability of water for irrigation can assessed not only from the total concentration of salt, but also from the type of salt and ions constituting it. It is then essential to study the parameters defining the characteristics of waters intended to irrigation.
Materials and Methods
Study area
The Cheffia dam on Bounamoussa wadi (river) is located 40 km south-east in the upstream of Annaba city, and 42 km south-west of El Tarf city in Cheffia town, Daïra of Bouteldja. It covers an area of 1000 ha. It is boarded from North by the communes of Cheffia and Asfour. In West by the commune of Asfour. In South by the municipalities of Hammam Béni Salah and Bouhadjar. And from East by Cheffia town (Fig. 1).
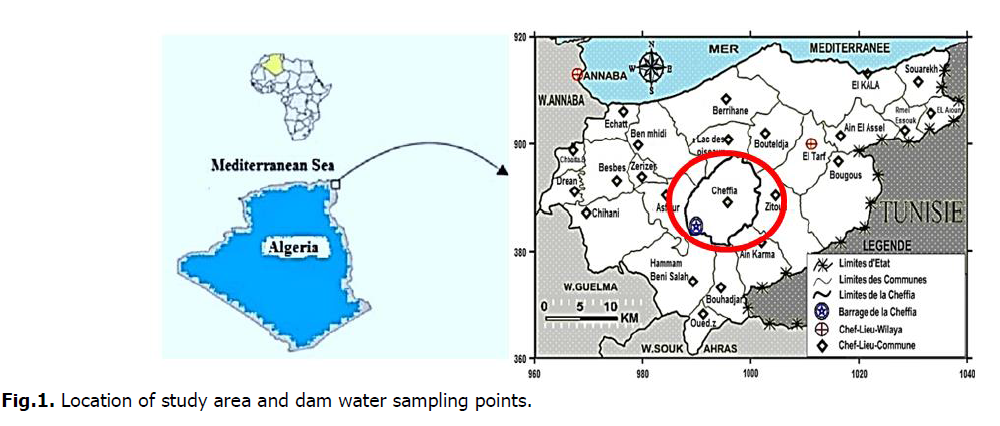
Fig 1. Location of study area and dam water sampling points.
Characteristics of Cheffia dam
Cheffia dam has a regulable water with a volume of 95 hm3. It supplies Bounamoussa perimeters with agricultural water in summer, the steel complex of El-Hadjar as well as the Annaba city and Bouhadjar with AEP. This dam is made of compacted soil with an upstream clay-sealing mask and alluvial recharge. The total volume of embankments is 1.3 million m3.
Cheffia dam is facing huge problems in water management from the point of view of distribution. The needs in agricultural water have never been satisfied at 100%, which justifies the failure in its management. The priority is given to the industrial sector and the AEP (cities of Annaba and El-Hadjar), and finally to the agricultural sector. The existence of Cheffia, and its dam location has served the need of water in three important sectors: a large city, that of Annaba, an industrial sector that of the steel complex of El-Hadjar and finally an agricultural sector, the irrigation perimeter of Bounamoussa.
Sampling and analysis
The sampling of Cheffia dam water was carried out along three campaigns (Febraury, March and April 2019). 27 samples were collected from 3 sampling points as shown in (Fig. 1). During these campaigns, samples were stored in polyethylene bottles with a capacity of 1.5 L, which have been rinsed three times with sampled water for a physicochemical analysis following accepted methods (Rodier, et al., 2009). The analyzed parameters were as follows: Temperature, pH, Electrical Conductivity EC, Salinity, Turbidity, Dissolved oxygen, Calcium (Ca2+), Magnesuim (Mg2+), Chloride (Cl–), Nitrate (NO3–), Nitrite (NO2-), Ammonium (NH4+), Phosphate (PO43–) and Iron (Fe) (Table 1). All of the sampling is usually conducted between 9:30 am and 11:00 am. After bottling and labeling, the sample should be placed in a cooler at 4°C to keep it cool. We located our sampling stations for the three samples as follows (Fig. 1):
Station 01: Southeast of the dam
Station 02: Depth of the dam
Station 03: North of the dam
| Parameters | Instrument/Method of analysis | Unitis |
|---|---|---|
| Temperature | Thermometer | C° |
| pH | pH meter | pH units |
| EC | Conductivity meter | µS/cm |
| Turbidity | Turbidity meter | NTU |
| DO | Oxymeter | % O2 |
| Salinity | Conductivity meter | g/l |
| Cl-, Ca++, Mg++ | Titrimetric method | mg/l |
| NO3-,NO2-,NH4+,PO4-3 | Spectrophotometer | mg/l |
| Fe, Na+ | flame spectrophotometer | mg/l |
Table 1. Different instruments or methods for parameters analysis.
For microbiological analysis, 27 samples were carried out using the standard Methods described by (APHA, 1999; Rodier et al., 2009). The study of bacteriological parameters was determined by the Millipore membrane filtration method (0.45 μm) and concerned with the quantification of faecal origin parameters: faecal coliforms (FC) and total coliforms (TC). This method utilizes elevated temperature incubation to distinguish faecal coliforms from the total coliforms. For faecal streptococci group, the counts were made with filtered membrane on Slanetz and Bartley Agar. The Results are expressed as colony forming units per unit of volume.
To characterize the microbiological contamination at the level of spring waters studied, we proceeded according to a method the calculation of the MCI. (Leclercq, 2001; Bovesse and Depelchin, 1980).
The Microbiological Contamination Index method is presented below (Bovesse and Depelchin, 1980). They include five quality classes corresponding to the generally accepted colors (Fig. 2).
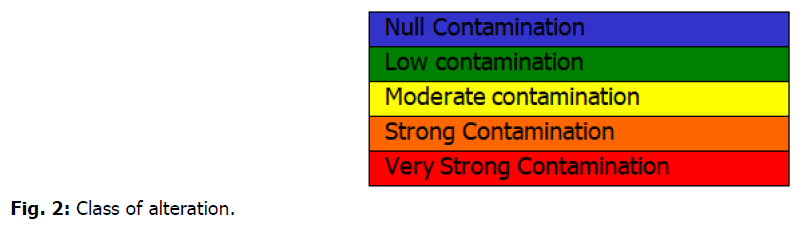
Fig 2. Class of alteration.
The principle is to divide the values of the bacteriological parameters into five classes (Table 2), then to determine, from one's own measurements, the corresponding class number for each parameter and then to take the average.
| Parameter/Class | Total Coliforms/ml | Faecal Coliforms/ml | Faecal Streptococci/ml |
|---|---|---|---|
| 5 4 3 2 1 |
<2000 2000-9000 9000-45000 45000-360000 >360000 |
<100 100-500 500-2500 2500-20000 >20000 |
<5 5-10 10-50 50-500 >500 |
Table 2. Quality grid (MCI).
MCI = (Total Coliforms + Faecal Coliforms + Faecal Streptococci)/3
MCI of 5.0 to 4.6: Null microbiological contamination;
MCI of 4.5 to 4.0: Low microbiological contamination;
MCI of 3.9 to 3.0: Moderate microbiological contamination;
MCI of 2.9 to 2.0: Strong microbiological contamination;
MCI of 1.9 to 1.0: Very strong microbiological contamination.
Sodium Adsorption Rate (SAR)
Alkalinity hazard is generally expressed by sodium adsorption rate (SAR). This parameter quantifies sodium ions proportion (Na+), calcium (Ca2+) and magnesium (Mg2+) in a water sample. It should be noted that Na+ plays a negative role in the soil since it reacts with the soil by decreasing its permeability and so by stopping waters circulation; its presence in the soil increases the clay particles volume, thereby leading to an obstruction of the pores between the particles. SAR calculation allows assessing the probable degradation of the soil structure and the alteration of its physical qualities. Sodium adsorption rate SAR has been calculated from the equation proposed by Richard (1954): 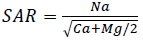
Results and Discussion
Physico-chemical characteristics of the water
The physico-chemical characteristics give an idea about the water quality in any water body.
Temperature (Tº)
The water temperature influences many other parameters. It is the first case for dissolved oxygen, which is essential for aquatic life. The higher water temperature the more the quantity of dissolved oxygen decreases. A too high water temperature can lead to dramatic situations like the lack of dissolved oxygen that can lead to: the disappearance of species, the reduction of the disappearance of species, the reduction of self-purification, the accumulation of foul-smelling deposits, accelerated growth of plants (including algae) (Arrigon, 1991). Water temperature measurements at Cheffia dam reached a minimum of 13.3°C (S3) during March and a maximum of 18.9°C at the same station during the month of April (Table 3).
| Sites | S1 | S2 | S3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| February | March | April | February | March | April | February | March | April | |
| TºC | 14.2 | 13.9 | 17.2 | 14.8 | 13 | 16.5 | 15.2 | 13.3 | 18.9 |
| pH | 7.18 | 7.20 | 7.01 | 7.05 | 7.30 | 7.20 | 7.02 | 7.35 | 7.71 |
| EC (µS/cm) | 372 | 306 | 310 | 392 | 292 | 378 | 453 | 382 | 398 |
| DO% | 71 | 78 | 77 | 75 | 78 | 79 | 72 | 70 | 81 |
| Salinity (g/l) | 0.2 | 0.15 | 0.18 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Turbidity (NTU) |
20 | 70 | 76 | 25 | 104 | 11 | 44 | 92 | 5.35 |
| Cl- (mg/l) | 26 | 2.5 | 22 | 42.84 | 2.5 | 42.1 | 38 | 2.5 | 42.5 |
| Ca++ (mg/l) | 59 | 10 | 42 | 40.5 | 10.5 | 59.5 | 44 | 10.22 | 43 |
| Mg++ (mg/l) | 10.2 | 9.1 | 9.77 | 22 | 8.2 | 2.5 | 7.10 | 7.10 | 7.70 |
| Na+ (mg/l) | 13.2 | 14.1 | 12.5 | 15.1 | 16.5 | 14.22 | 22.5 | 32.2 | 25.7 |
| NO3- (mg/l) | 0.8 | 0.7 | 0.8 | 0.6 | 0.2 | 0.9 | 0.3 | 0.5 | 0.8 |
| NO2- (mg/l) | 0.09 | 0.08 | 0.12 | 0.01 | 0.05 | 0.01 | 0.02 | 0.02 | 0.15 |
| NH4+ (mg/l) | 0.09 | 0.19 | 0.003 | 0.28 | 0.28 | 0.008 | 0.004 | 0.08 | 0.004 |
| PO4- (mg/l) | 0.02 | 0.001 | 0.01 | 0.001 | 0.009 | 0.003 | 0.01 | 0.005 | 0.004 |
| Fe (mg/l) | 0.01 | 0.4 | 0.9 | 1.2 | 5 | 0.09 | 0.1 | 0.9 | 0.4 |
Table 3. Descriptive study for water chemistry
Potential of hydrogen (pH)
The pH (hydrogen potential) measures the concentration of H+ ions in water. It translates the balance between acid and base on a scale of 0 to 14 being the pH of neutrality. This parameter characterizes a large number of physico-chemical equilibrium and depends on multiple factors including the origin of the water. The pH must be measured on the ground and must be between 6.5 and 9 (Devillers, et al., 2005). The pH of the dam water usually varies between a minimum value of 7.01 (S1) and a maximum of 7.71 (S3). The majority of this pH is slightly basic (Table 3). Our pH levels are almost similar to those obtained in Bounamoussa River which varied between 6.94 and 8.02 (Ramdani and Laifa, 2017).
Electrical conductivity (EC)
Conductivity measures the ability of water to conduct an electric current between two electrodes. Most substances dissolved in water are in the form of electrically charged ions. Thus, measuring conductivity allows us to evaluate the amount of dissolved salts in the water (Devillers, et al., 2005). The obtained results in the study area show that the electrical conductivity of water varies between 292 μS/Cm (S2) in March and a maximum value of 453 μS/Cm (S3) in February (Table 3). Our results show that EC values do not exceed the permissible limit (1500 μS/cm) set for drinking water (WHO, 2004).
Dissolved Oxygen
Oxygen is one of the particularly useful parameters for water and it is an excellent indicator of its quality. It is one of the most sensitive parameters to pollution (Makhoukh, et al., 2011). The observed dissolved oxygen content of the dam water rises to significant concentrations up to 81% O2 (S3) during the month of April (Table 3).
Salinity
Salinity is an ecological factor specific to aquatic biotopes (but also to soils) which characterize their salt content (NaCl) and other salts dissolved in water. Furthermore, any untimely modification of the salinity due to the action of man can present a frightening impact on the aquatic biotopes concerned (Rodier, et al., 2009). The salinity content of the dam water varies between 0.15 g/l at sites (S1) in March (minimum value), and a maximum value 0.2 g/l for (S1, S2 and S3) in February, while it stabilizes for the other two stations during the 3 months (Table 3). This value is lower than the average salinity value obtained in the study of El Mehdi, et al, (2009).
Turbidity
Turbidity reflects the presence of particles in suspension in water (organic debris, mud, microorganisms, etc.). High turbidity can allow microorganisms to adhere to suspended particles and reduce the light that aquatic plants use for photosynthesis. Turbidity is expressed in turbidity units (NTU) and should be less than 5 NTU (Bengoumi, et al., 2013). For all analyzed samples of water, the turbidity varies between a minimum value of 5.35 NTU (S3) during April and a maximum value of 104 NTU (S2) in March (Table 3) which is higher than the standard permissible limits set by Algeria and the World Health Organization. These high values are due to the high presence of organic and inorganic materials such as silt and Sediment.
Cations calcium and magnesium (Ca+2 and Mg+2)
Both calcium and magnesium are essential minerals and beneficial to human health in several aspects (Boumaraf, et al., 2017). Inadequate intake of either nutrient can result in adverse health consequences (WHO standard). The results of cations Ca+2 and Mg+2 were found to be in the range of 10 to 59 mg/l and 2.5 to 22 mg/l, respectively (Table 3). The calcium levels are mostly below the maximum acceptable concentration (200 mg/l) set by Algeria and WHO for drinking water. The magnesium values are in agreement with the Algerian standard (JORA, 2011) which is set a maximum value of 150 mg/l.
Chlorides (Cl-)
The chloride content of water is extremely different and it is mainly related to the nature of soil through which it flows. The WHO recommends a guide value of 200 mg/l for the chloride content in water intended for human consumption. The contents fluctuate between a minimum value 2.5 mg/l at the station 1 (March) and a maximum value of 42.84 mg/l at (S2) depth of the dam during February (Table 3). Concentrations of all these anions were found to be under permissible limits set by Algeria and WHO for drinking and irrigation water (Cl-<250 mg/l). Our chlorides levels are similar to those obtained in the Guebli River (Boudeffa et al., 2020).
Ammonium (NH4+)
Ammoniacal salts are pollutants that come from domestic effluents, agricultural fertilizers and some industrial and industrial units. In groundwater, NH4+ is frequently present without being an indication of pollution. On the other hand in surface waters NH4+ is present only in polluted waters (Valiron, 1994). The water of Cheffia dam presents an important content of ammonium arrives until 0,28 mg/l and that is in the depth of the dam (S2) in the months February and March (Table 3). The WHO recommends 0.5 mg/l as a limit value, for the analyzed samples the ammonium contents in all samples are within the standards of potability vary between 0.013 mg/l and 0.28 mg/l.
Nitrate (NO3-)
Nitrates (NO3-) are produced from the oxidation of organic nitrogen from fertilizer leaching, urban, industrial leaching of fertilizers and urban industrial waste, and are therefore naturally present in soil and water. Nitrates are one of the main plant nutrients. Their presence, together with other nutrients, stimulates the growth of aquatic plants (Kahoul and Touhami, 2014). The average concentrations of NO3– ions are lower than 1.1 mg/l during the 3 months of (February, March and April) (Table 3). These values were at the lower limit of the range for nitrate ion concentrations in the unpolluted surface water. These results indicate that the studied waters of Cheffia dam are not at risk of pollution from nitrate ions.
Nitrite (NO2-)
Nitrite (NO2), in the form of nitrate, occurs naturally in soil, water and plants, but usually in small amounts plants, and typically in small amounts. The higher the nitrate content of the water, the greater the risk of consuming nitrites is greater, because nitrates are converted into nitrates by the chemical reduction (removal of oxygen). A very high concentration of nitrite in the body can lead to serious diseases (especially cyanosis) (Toumi, et al., 2016). The average concentrations of nitrite ions were smaller than ammonium ions. The results of the nitrite content are low during the three months of (February, March and April) (Table 3). According to WHO standards, our water corresponds to good quality (NO2- ≤ 0.2 mg/l).
Phosphate (PO4-3)
The natural presence of phosphates (PO4-3) in waters is linked to the characteristics of the ground crossed and to decomposition of organic matter (Kumar et al., 2017). The presence of phosphates in natural waters with concentrations above 0.2 mg/l indicated pollution by synthetic detergents, as well as by runoff water (Kumar et al., 2018). According to the results, the dam water has very low phosphate concentrations which are <0.025 mg/l. Compared to the WHO standards (PO4-3<0.1 mg/l), most of the samples correspond to good quality waters (Table 3).
Iron (Fe)
The presence of iron could be due to the contact of rocks, a high concentration of iron affects the taste and has adverse effects on domestic uses which promotes the growth of iron bacteria (Kumar et al., 2017). The iron content of the dam water varies from a minimum value of 0.01 mg/l (S1) in February to a maximum value of 5 mg/l (S2) in the month of March. The waters from depth (S2) have higher iron contents than the values limited by the world standards at 0.3 mg/l (Table 3). Accidental overdose of iron can cause damage to the liver, heart and joints. It can also lead to hematochromatosis (heavy iron deposition in tissues). Also cerebral edema, hypovolemia, hypotension and sometimes even spontaneous abortion or premature delivery (Hlavackova, 2005).
Water suitability for irrigation
SAR values calculated allow isolating only one category: excellent quality water with low-alkalinization hazard. According to Riverside diagram established by surface waters of study area ) which shown that they belong to classes (C2-S1) (Fig. 3). These classes were as medium to good quality for irrigation and as water for use with sensitive plants and in poorly drained soils and for sensitive plants (fruit trees).
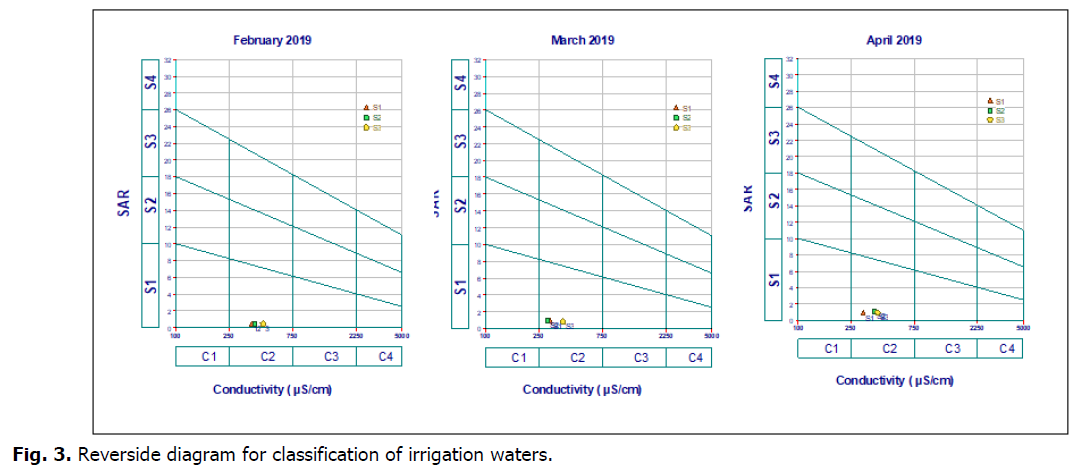
Fig 3. Reverside diagram for classification of irrigation waters.
Bacteriological analysis of dam waters
Total coliforms
Based on the analysis of data and the interpretation of total coliforms percentage, fecal coliforms and streptococci; our results show values during the three months of study (February, March and April) as follows. The majority of the waters are dominated by total coliforms during the month of April S1 (500TC), S2 (520TC) and S3 (460TC). On the other hand, a decrease is observed during the month of February S1 (50TC), S2 (105TC) and S3 (82TC) (Table 4). The results of water bacteriological analysis show that all samples collected during the month of April were polluted by the presence of coliforms. These are indicators of faecal contamination of the water. The contamination of these waters by total germs could be due to the poor protection of the dam and the ignorance of the elementary rules of hygiene.
| Sites | Months | TC/ml | FC/ml | FS/ml | Index | Contamination |
|---|---|---|---|---|---|---|
| S1 | February March April |
0.50 1.20 5.00 |
0.50 1.50 0.49 |
0.42 0.42 0.63 |
5 5 5 |
Null Null Null |
| S2 | February March April |
1.05 4.50 5.20 |
0.90 2.50 0.40 |
0.44 0.85 0.44 |
5 5 5 |
Null Null Null |
| S3 | February March April |
0.82 0.95 4.60 |
0.50 1.00 0.50 |
0.44 0.18 1.44 |
5 5 5 |
Null Null Null |
Table 4. Values of indices and classes of contamination for dam waters.
Faecal coliforms and Faecal Streptococci
Our results indicate significant values of faecal coliforms during the month of March with an average concentration of (166FC) (Table 4). Significant values of faecal Streptococcus are observed during the study period: S1 (Avg. 49 FS), S2 (Avg. 57FS), S3 (Avg. 68 FS) (Table 4). The rate of faecal coliforms is 250 colonies/100 ml. These values are higher than the WHO standards (S2 during the month of March). The high presence of faecal coliforms in the dam water presents a potential risk for health and human consumption. The discharge of wastewater from several residential units into the dam may also be an important source of streptococci in the water that participate in the contamination of this site. However; a strong faecal contamination in low water period where bacterial multiplication is more favored by temperature, basic pH and alkaline environment which was supported by other works (Aboulkacem, et al., 2007; El-Addouli, et al., 2011; Lamrani, et al., 2011). The high concentrations of total coliforms, faecal coliforms and faecal streptococci suggest the possible existence of the wastewater discharge at this dam.
Application of the microbiological contamination index method
For a more significant interpretation, we have chosen two results from the different bacteriological parameters of each month for all sites (Table 4).
According to the Table 4, and during the month of February we have noticed that at the level of all the sites the microbiological contamination is null. According to the results of April, we notice a null contamination but with an early more or less high of total coliforms at the level of S1 and S2, with respectively 500 and 520 germs/100 ml, seen the norms of WHO. This does not translate a danger but rather a water to be watched.
During the month of March and following the results of Table 4, the contamination index is null but with an increase of faecal coliforms at the site S2 level whose early is 250 germs/100 ml, respectively, due to runoff and gravity runoff of the mountains.
Finally, for the faecal Streptococci and according to the results, we note a null contamination consequently water of good bacteriological quality.
The low values of this index are explained by a low total coliform count and faecal coliforms and faecal streptococci in the studied stations.
Conclusion
At the end of this study, we have assessed surface waters quality of Cheffia dam, used for irrigation by the application of quality parameters of waters intended to agricultural activity, such as sodium adsorption rate (SAR).
At the physicochemical level, the present study showed that the water samples were great in quality and below the concentrations permitted by the World Health Organization for drinking water. The faecal contamination germs counted in Cheffia dam water are due to the domestic wastewater discharges and the use of chemical and natural fertilizers (animal droppings). According to the microbiological contamination index and during all our period of study, the contamination is null. It emerges that the studied water stations is of an excellent bacteriological quality.
According to the means values of SAR, all sampled surface waters are of good quality for agriculture and that these waters are fit for irrigation of most cultures, but attention should be paid to leaching, difficult in marginally permeable soils, and to drainage.
Acknowledgment
We are very grateful to my husband and my colleague Dr. Bergal for the help and support they have given me.
References
Aboulkacem, A., Cahlaoui, A., Soulaymani, F., Benali, D., Rhazi, F. (2007). Etude comparative de la qualité bactériologique des eaux des Oueds Boufekrane et Ouislane à la traversée de la ville de Meknès (Maroc). Review Microbiology Ind San et Environment, 1:10-22.
APHA. (1999). Standard methods for the examination of water and wastewater. American Public Health Association "APHA", Washington DC.
Arrigon, J. (1991). Aménagement piscicole des eaux douces. 7éme édition, Lavoisier. Paris, p:639.
Barbault, R. (2007). Développement et diversité écologique: liens et connexions?. In Mollard A., Sauboua E., Hirczak M. Territoires et enjeux du développement régional: éditions Quae c/o INRA, Versailles.
Bengoumi, D., Chahlaoui, A., El Moustaine, R., Belghiti, L., Samih, M. (2013). Typology of well water quality used for poultry watering (Gharb and Meknes Morocco), Science. Lib, 5:1-23.
Benrabah, S., Attoui, B., Hannouche, M. (2016). Characterization of groundwater quality destined for drinking water supply of Khenchela City (eastern Algeria). Journal of Water and Land Development, 30:13-20.
Boudeffa, K., Fekrache, F., Bouchareb, N. (2020). Physicochemical and biological water qualityassessment of the guebli river, northeastern Algeria. Rasayan Journal of Chemistry, 13:168-176.
Boumaraf, W., Djamai, R., Djabali, N. (2017). Hydro-chemical characterization of an aquatic ecosystem water of El Kala national park (Case of Oubeira Lake, Northeast Algeria). International Journal of Bioscience, 11:59-67.
Bovesse, M., Depelchin, A. (1980). Cartography of the pollution of the watercourses of the province of Namur: bacteriological analyses.
Devillers, J., Squilbin, M., Yourassowsky, C. (2005). Qualité physico-chimique et chimique des eaux de surface. Institut Bruxellois pour la Gestion de l’Environnement, pp:1-4.
El Addouli, J., Chahlaoui, A., Berrahou, A., Hafi, A., Ennabili, A. (2010). Approche de la qualité biologique de l’Oued Ouislane, au voisinage des effluents bruts de la région de MEKNES. Larhyss Journal, 9:21-33.
El Mehdi, T., Lahcen, B., Chakib, N., Kawtar, F.B. (2009). Caractérisation des effluents liquides de l’hôpital Al Ghassani, CHU Hassan II de Fès, Maroc. Les effluents liquides des établissements de santé: état des lieux et perspectives de gestion. Rev Hospi France, 714:47-50.
Guiraud, M. (1998). Microbiologie alimentaire. Techniques d'analyse microbiologiques. Ed, Dunod.
Hlavackova, P. (2005). Evaluation du comportement du cuivre et du zinc dans une matrice de type sol à l’aide de différentes méthodologiques. Institut National des Sciences de Lyon, Ecole doctorale:chimie de Lyon, p:202.
Kahoul, M., Touhami, M.I. (2014). Evaluation de la qualité physico-chimique des eaux de consommation de la ville d’Annaba (Algérie). Larhyss Journal, 19:129-138.
Kumar, S.K., Babu, S.H., Rao, P.E., Selvakumar, S., Thivya, C., Muralidharan, S., Jeyabal, G. (2017). Evaluation of water quality and hydrogeochemistry of surface and groundwater, Tiruvallur District, Tamil Nadu, India. Applied Water Science, 7:2533-2544.
Kumar, D., Kumar, V., Kumari, S. (2018). Study on water quality of Hindon river (tributary of Yamuna river). Rasayan Journal of Chemistry, 11:1477. DOI:10.31788/RJC.2018.1143075.
Lamrani, H., Chahlaoui, A., El addaouli, J., Ennabili, A. (2011). Evaluation de la qualité physicochimique et bactériologique de l’Oued boufekrane au voisinage des effluents de la ville de Meknès (MAROC). Science Lib, 3:111-112.
Leclercq, L. (2001). Running waters: characteristics and means of study, in wetlands. Proceedings of the colloquia organized in 1996 by the Ministry of the Walloon Region in the framework of the World Year of Wetlands, Legs, Walloon Region, DGRNE, pp:67-82.
Makhoukh, M., Sbaa, M., Berrahou, A., Clooster, M. (2011). Contribution a l’etude physico-chimique des eaux superficielles de l’oued Moulouya (Maroc oriental). Larhyss Journal, 9:149-169.
Official Journal of the Algerian Republic (JORA). (2011). Executive decree n° 11-125 of 17 Rabie Ethani 1432 corresponding to March 22, 2011 relating to the quality of water for human consumption. Official Printing Office. Les Vergers: Bir-Mourad Raïs, Algiers.
Olivaux, Y. (2007). La Nature De L’eau, Édition Marco Pietteur, Collection Résurgence.
Ramdani, H., Laifa, A. (2017). Physicochemical quality of Wadi Bounamoussa surface waters (Northeast of Algeria). Journal of Water and Land Development, 35:185-191.
Richards, L.A. (1954). Diagnosis and improvement of saline and alkali soils. Agricultural Hand book, N° 60, USDA, Washington D.C. p:160.
Rodier, J., Legube, B., Merlet, N. (2009). L'analyse de l'eau, 9th edition, Ed., Dunod, p:1579.
Toumi, A., Reggam, A., Alayat, H., Houhamdi, M. (2016). Physico-chemical characterization of the waters of the lacustrine ecosystem: case of the Lac des Oiseaux (Extreme NE-Algeria). Journal of Material Environmental Science, 7:139-147.
Valiron, F. (1994). Gestion des résidus de l’assainissement. In: Mémento du gestionnaire de l’alimentation en eau et de l’assainissement, Tome 2: Assainissement urbain. Paris: TEC & DOC, pp:621-634.
World Health Organization. (2004). Guidelines for Drinking Water Quality. WHO, Geneva Recommendation.
Author Info
W. Boumaraf1*, A. Bergal1 and A. Delimi1,22Laboratory of Biodiversity and Ecosystem Pollution, Faculty of Natural and Life Sciences, University of Chadli Bendjedid El Tarf, Algeria
Citation: Boumaraf, W., Bergal, A., Delimi, A. (2022). Assessment of water quality of the Cheffia Dam in Algeria based on microbiological quality index and suitability for irrigation. Ukrainian Journal of Ecology. 12:36-43.
Received: 30-Jan-2022, Manuscript No. UJE-22-52853; Accepted: 25-Feb-2022, Pre QC No. P-52853; Editor assigned: 01-Feb-2022, Pre QC No. P-52853; Reviewed: 14-Feb-2022, QC No. Q-52853; Revised: 19-Feb-2022, Manuscript No. R-52853; Published: 28-Feb-2022, DOI: 10.15421/2022_342
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
