Research - (2022) Volume 12, Issue 5
Antibacterial activity and identification by GC/MS of the chemical composition of essential oils of Juniperus phoenecea and Juniperus oxycedrus L. from Western Algeria: Tiaret province
A.H.A. Boukhaloua1,4*, M. Berrayah2, F. Bennabi1,4, A. Ayache1,3 and F. Abdeldjebar1,4Abstract
Extraction by hydrodistillation and characterization by GC/MS of essential oils from the aerial part of the two species of the Juniperus genus: Juniperus phoenecea and Juniperus oxycedrus, harvested in the province of Tiaret; showed the presence of seventeen compounds in J. oxycedrus oil, the main constituents of which were: hydrocarbon monoterpenes (69.2%), α-pinene (29.1%), β-copaene (19.3%), β-pinene (17.6%), limonene (12.1%), α-fenchene (5.1%). The J. phenecea manifested thirteen components, the main constituents of which were: β-pinene (35.7%), α-pinene (21.3%), β-coepane (8.3%) and p-cymene (6, 6%) and segmaterpinene (3.7%). While the antimicrobial activity of the two essential oils carried out on bacteria referenced (Gram+) and (Gram-); showed good activity on all the bacteria tested, with the exception of the Pseudomonas strain which proved to be very resistant to Juniperus phoeneceae while the Juniperus oxycedrus oil is active on all the bacterial strains tested unlike the strain staphylococcus.
Keywords
Juniperus phoenecea, Juniperus oxycedrus, Essential oil, Chemical composition, GC/MS, Antimicrobial property.
Introduction
Junipers are important precursors in the dynamics of forest groupings in Mediterranean vegetation, but also develop under extreme ecological conditions (Quezel et Medail, 2003). The genus Juniperus is an integral part of arid and semi-arid ecosystems in the northern hemisphere (Farjon, 1992; Adams, 2008). In the Algerian flora, five species are present: Juniperus oxycedrus L., Juniperus Sabina L., Juniperus thurifera L., Juniperus communis L. and Juniperus phoenecea L. (Harhour, 2018; Quezel et Santa, 1963).
Juniperus phoenecea L., also called red cedar, is an evergreen coniferous shrub or small tree belonging to the Cupressaceae family. In Algeria, it covers an area of 227,000 ha (Louni, 1994). It is considered an important medicinal plant widely used in traditional medicine. Its leaves are used as a decoction to treat rheumatism and diabetes. In powder form it is used against bronchopulmonary diseases and as a diuretic (Bellakhder, 1997). The mixture with berries is used as an oral hypoglycaemic agent (Amer et al., 1994). Dried and powdered fruits can cure skin ulcers and abscesses (Le Floc'h, 1983).
Juniperus oxycedrus L. called Taga in Algeria (Quezel et Santa, 1962) is distributed in different parts of the world, including the Mediterranean regions. It can be found in the Tell, mainly associated with holm oaks (Quercus ilex L.), cork oaks (Quercus suber L.), and even Aleppo pines (Pinus halepensis), and on mountainous massifs. Not very demanding in terms of soil, it can be found mainly on limestone, at meso- and supra-Mediterranean levels, in a sub-humid bioclimate. It can appear very locally in semi-arid bioclimates (Boudy, 1950). This plant is used in traditional Algerian medicine as a diuretic, stomach tonic and disinfectant (Baba Aïssa, 1991).
Materials and Methods
Plant material
Specimens of Juniperus phoenicea were collected from Ain Bezzez in the Naddor massif (eastern of Tiaret province) and Juniperus oxycedrus was collected from the western forest of Tiaret. Fruits and leaves were collected during their flowering period in January 2022 (Fig. 1-3).
Fig 1: Location of the sampling sites.
Fig 2: Juniperus oxycedrus.
Fig 3: Juniperus phoenecea.
Extraction of the essential oils
All samples were air-dried at room temperature in the dark and stored in paper bags in a cool, dry and dark place. Once the plants were dried, we hydro distilled them for 3 h using a Clevenger. The oil obtained was collected and dried by anhydrous sodium sulphate and collected in opaque glass bottles stored at 4-5°C. The yield was calculated on the basis of the dry weight of the samples in percent.
Analysis of essential oils
The essential oils of Juniperus phoenecea and Juniperus Oxycedrus were analysed by Heweltt Packard 6890 N gas chromatography, driven by a HP5MS capillary column of 30 m length, 0.25 mm internal diameter and 0.25 µm film thickness. The analyses were carried out at isothermal temperatures, programmed at 60°C for 8 min, and then at a variable increase between 2°C and 250°C for 10 min. This operation was 113 minutes. The volume injected 0.2 µl at 250°C with one analysis mode. Scan Tic (35 to 600) and a solvent delay of 3.5 min at 280°C. The gas chromatograph is coupled to an Agilent 5973 mass spectrometer. The helium carrier gas was of N6.0 purity with a flow rate of 0.8 ml/min managed in split 1:100 mode. Ionisation was performed by electron impact at 70 ev, with an ion source temperature of 270°C. The results obtained were processed by Workstation 8 software, supported by the software's internal database and by the NIST and AMDIS database. A series of alkanes was injected under the same operating conditions in order to calculate the retention index for each peak detected.
Antimicrobial activity
The sensitivity of microbial strains to essential oils was studied using the disc diffusion method against bacterial strains (Perez et al., 1990). The standards obtained were Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 11778, Escherichia coli ATCC 25922, Pseudomenas aurus ATCC 27853 and a yeast Candidas albicans ATCC 10231. Whatman sterile discs (6 mm diameter) were impregnated with 10 μL of essential oil and placed on the central surfaces of Müller Hinton agar inoculated with a bacterial or fungal suspension. After diffusion, the petri dishes were incubated at 37 C for 24 h for bacteria and at 25 C for 48 h for yeast, the diameters of the clear inhibition zones were measured with a caliper. All tests were performed in triplicate and the antibacterial sensitivity of the oils was expressed as the average of the inhibition diameters produced (in mm).
Determination of the minimum inhibitory concentration (MIC)
MIC is the lowest concentration of essential oil at which no bacterial entrainment is observed. The calculation of MIC was performed by the microdilution method (Andrews, 2001), using 96-well plates. A fixed volume of Mueller Hinton broth placed in each well followed by the presence of each strain tested and a dilution of the essential oil, with the exception of the positive control consisting of 100ul MH broth with 20 ul inoculum and the negative control consisting of MH broth alone. Plates were incubated for 24 hours, distinguishing susceptible from resistant strains for each concentration by the presence (+) or absence (-) of a deposit at the bottom of the well and the cloudiness.
Results and Discussion
Chemical composition of essential oils
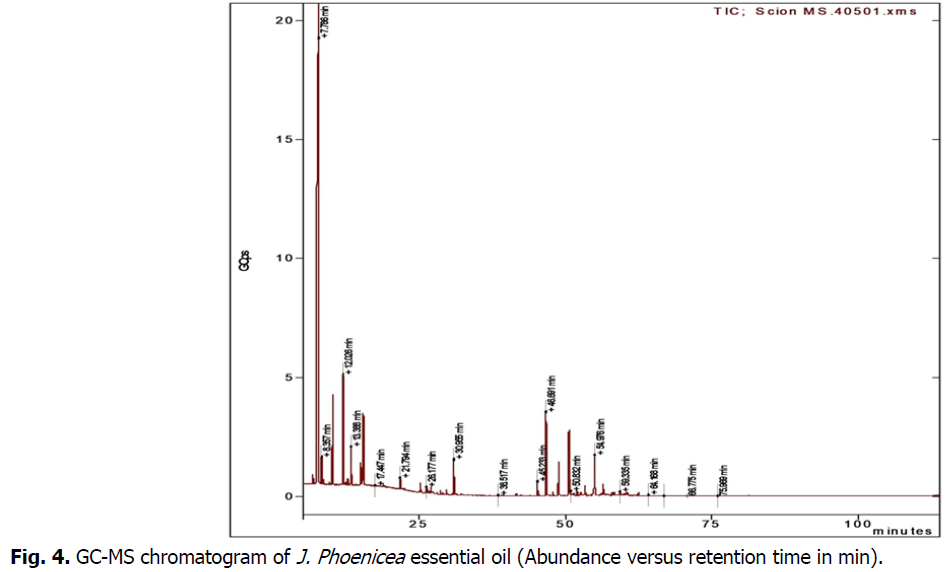
GC-MS analysis (Table 1) (Fig. 4) of the essential oils obtained from J. phenecea with a yield of 0.8% showed the presence of seventeen identified compounds representing 90.3% of the total oil composition. The main compounds were β-pinene (35.7%), followed by α-pinene (21.3%) beta-copaene (8.3%), p-cymene (6.6%), γ-terpinene (3.7%), γ-muurlene (3.6%), (E)-β-caryophyllene (2.1%).
| S. No | Compoundsa | RIab | RIac | % d | Identificatione |
|---|---|---|---|---|---|
| 1. | α –pinene | 939 | 942 | 21.3 | RI,MS |
| 2. | α –Fenchene | 947 | 947 | 0.1 | RI,MS |
| 3. | Camphene | 948 | 946 | 1.6 | RI,MS |
| 4. | β –Pinene | 978 | 975 | 35.7 | RI,MS |
| 5. | α- Phelllandrene | 1004 | 1001 | 1.3 | RI,MS |
| 6. | γ-Terpinene | 1058 | 1055 | 3.7 | RI,MS |
| 7. | p-cymenene | 1064 | 1067 | 6.6 | RI,MS |
| 8. | Terpinolene | 1087 | 1089 | 0.9 | RI,MS |
| 9. | α – Campholenal | 1125 | 1122 | 0.7 | RI,MS |
| 10. | Camphor | 1138 | 1142 | 1.3 | RI,MS |
| 11. | α- Terpineol | 1189 | 1193 | 1.6 | RI,MS |
| 12. | (E)-β- Caryophellene | 1421 | 1419 | 2.1 | RI,MS |
| 13. | β- Copaene | 1435 | 1431 | 8.3 | RI,MS |
| 14. | γ-Muurolene | 1473 | 1474 | 3.6 | RI,MS |
| 15. | 4-Epi-Cubebol | 1493 | 1490 | 0.1 | RI,MS |
| 16. | γ-Cadinene | 1517 | 1514 | 0.1 | RI,MS |
| 17 | Oxyde de Caryophellenes | 1582 | 1583 | 1.3 | RI,MS |
| Total identification % | 90.3 | ||||
| Monoterpene hydrocarbons | 71.2 | ||||
| Oxygenated monoterpenes | 3.6 | ||||
| Sesquiterpene hydrocarbons | 14.1 | ||||
| Oxygenated sesquiterpenes | 1.4 | ||||
Table 1. Chemical composition of essential oil of analysis Juniperus Phoenecea.
Fig 4: GC-MS chromatogram of J. Phoenicea essential oil (Abundance versus retention time in min).
Many studies have been carried out on the chemical composition of J. phenecea oil in different regions. Derwich et al, 2001 in Tunisia, found that the yield of essential oil of J. phoenecea was 1.62% and the main compound in the aerial parts was α-pinene (49.15%), followed by α-phyllandrene (7.39%), mycene (5.24%), β-pinene (3, 58%), linalool (2.54%), piperitone (1.56%), γ-terpinene (1.28%), trans-pinocarveole (1.23%) ρ-cymene (1.10%), α-terpineol (1.02%) and γ-cardinene (1.01%). By comparison of our results, we noted, the absence of α-pherlandrene and the presence of a β-pinene component (35.7%) as the first major component; however, β-myrcene and caryophyllene oxide and 4-Epi-Cubebol shows a lower yield in the order (1.3% and 0.1%, respectively). This variation could be due to geographical and bioclimatic factors of the provinces. On the other hand (Fouad et al., 2011) in Morocco revealed the presence of sixty-three volatile compounds that represent 52-92% of the total petroleum compositions. The main monoterpenes were α-pinene (26.7-78.7%) and δ-3carene (7.6-15.4%). According to (Ramdani et al., 2013) collected from Boutaleb (Setif), Boussâada (M'sila), Menâa and T'kout (Batna), and Elhadjab (Biskra) showed that the average yield of essential oil from the samples is 0.82%, The chemical composition of J. Phoenecea is dominated by the presence of a major product, α-pinene (36.3-55.9%). Three components are represented with high concentrations, terpinolene (0-13%), Δ3-carene (0-12.4%) and β-phellandrene (0-7.3%). Another work showed that the main components of J. phenecea leaf oil (Abdeli et al., 2018) were mainly composed of β-phellandrene (44.9%), α-pinene (20.3%), myrcene (8.2%), α-phellandrene (4.5%) p-cymene (3.0%) and limonene (2.5%). Harhour et al., 2018; found that the main constituents α-Pinene (67.7 4.3%) were the main compound oils, followed by δ-3-carene (26.8 2.3%) and α-cedrol (7 1.1%). From the comparison study between the cited works and the results found, we note the presence of several similar products at comparable concentrations, the only difference being the presence in the extracted oils of β-Pinene as the majority product, unlike the other studies where the majority product is α-Pinene.
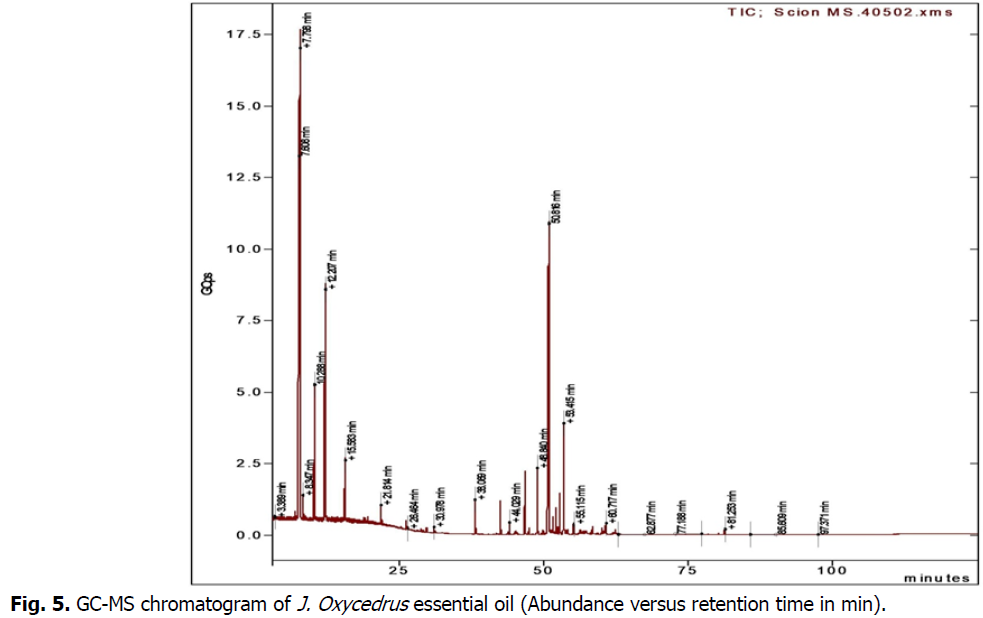
GC-MS analysis (Table 2) (Fig. 5) of J. Oxycedrus oil with a yield of 1.8% showed the presence of thirteen identified compounds representing 90.3% of the total oil composition. The main compounds were α-pinene (29.1%), β-copaene (19.3%), β-pinene (17.6%), limonene (12.1%), α-fenchene (5.1%), (E)-β-caryophyllene (3.7%) and β-ylangene (2.5%). Many studies have identified the chemical composition of J. oxycedrus oil in different regions. J. oxycedrus from Djelfa (Algeria) (Dob et al., 2006) showed that the main components were trans-pinocarveol (7.0%), cis-verbenol (6.3%) and manoyl oxide (6.0%), respectively. The main constituents identified by Fadel et al. in 2019 were manoyl oxide (23.5%), pentadecane-2-enene 6Z (12.6%), abietatriene (8.0%), abieta-8,11,13-triene-7-one (6.5%), cubebol (4.6%), epi-torilenol (3.8%) and a-cadinol (2.6%). Regarding the work of (Boudiba et al., 2021), the main constituents found in the fruit oil were α-pinene (57.5%), α-amorphene (9.0%), β-myrcene (8.0%), δ-cadinene (1.7%), manoyloxide (1.5%) and zonarene (1.4%). Several studies on the same plant from different parts of the world collected in El pennon (Spain) (Adams, 1998) revealed that the main compounds were a-Pinene (41.3%) (a-Phellandrene (8.2%) p-Cymene (6.2%). From Crysortis (Greece) (Adams et al., 1999) found that the main components a-Pinene (42.7%), Limonene (17.1%), d-3-Carene (13.7%). Angioni et al., 2003; from Sardinia (Italy) the main constituents were a-Pinene (85.6%) d-3-Carene (2.8%) c-Terpinene (1.4%). According to (Loizzo et al., 2007); J. oxycedrusssp. oxycedrus berry oil was characterised by high contents of α-pinene (27.4%) and β-myrcene (18.9%). Other important compounds were α-phellandrene (7.1%), limonene (6.7%) and δ-cadinene (2.2%). For Alan et al., 2016; the main compounds in the oil of J. oxycedrus from Turkey were manoyl oxide (32.8%) and caryophyll oxide (11.9%) in the leaf oil, myrcene (44.6%), a-pinene (19.9%) and D-germ (15.5%) in the berry oil, and manoyl oxide (35.4%) and caryophyll oxide (16.8%) in the twigs.
| S.No | Compoundsa | RIab | RIac | %d | Identificatione |
|---|---|---|---|---|---|
| 1. | α-Thujene | 932 | 929 | 2,3 | RI,MS |
| 2. | α–pinene | 939 | 937 | 29,1 | RI,MS |
| 3. | α-Fenchene | 947 | 945 | 5,1 | RI,MS |
| 4. | Camphene | 948 | 950 | 0,9 | RI,MS |
| 5. | Sabinene | 971 | 970 | 2,1 | RI,MS |
| 6. | β-pinene | 978 | 976 | 17,6 | RI,MS |
| 7. | Limonene | 1021 | 1020 | 12,1 | RI,MS |
| 8. | β- Ylangene | 1375 | 1373 | 2,5 | RI,MS |
| 9. | (E)- β- Caryophellene | 1421 | 1419 | 3,7 | RI,MS |
| 10. | β- Copaene | 1435 | 1137 | 19,3 | RI,MS |
| 11. | γ-Muurolene | 1473 | 1471 | 1,6 | RI,MS |
| 12. | 4-Epi-Cubebol | 1493 | 1492 | 0,3 | RI,MS |
| 13. | γ-Cadinene | 1517 | 1515 | 0,1 | RI,MS |
| Total identification % | 96.7 | ||||
| Monoterpenehydrocarbons | 69.2 | ||||
| Sesquiterpenehydrocarbons | 27.2 | ||||
| Oxygenatedsesquiterpenes | 0.3 |
Table 2. Chemical composition of essential oil of analysis Juniperus Oxycedrus.
Fig 5: GC-MS chromatogram of J. Oxycedrus essential oil (Abundance versus retention time in min).
The antimicrobial activity
The results of the aromatogram of J. phoenecea and J. oxycedrus essential oils against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus and Candida Albicans: ATCC10231 are shown in Table 3.
| Microorganisms | EO J. Phenecea | EO J. Oxycedrus |
|---|---|---|
| Strains | D (mm) | D (mm) |
| Escherichia coli | 20 mm | 30 mm |
| Staphylococcus aureus | 47 mm | 20 mm |
| Bacillus cereus | 20 mm | 25 mm |
| Pseudomonas aeruginosa | 28 mm | 21 mm |
| Candidas abicans | 28 mm | 30 mm |
Table 3. Antibacterial activity by zone of inhibition of J. phoenecea and J. oxycedrus oils.
J. phoenecea oil exerts strong antibacterial activity against the referenced strains tested. It was found that Staphylococcus aureus ATCC11778 seems to be extremely most sensitive to J. phoenecea oil tested with a diameter of 47 mm and moderately sensitive with our oil against strains (Pseudomonas aeruginosa: ATCC10231 and Candida albicans yeast: ATCC10231) with a diameter of 28mm and weakly sensitive with Escherichia coli: ATCC25922 and Bacillus cereus: ATCC25923 with a diameter of 20 mm.
The results are read as follows (Djabou et al., 2013).
• Resistant (-): diameter ≤ 8 mm
• Moderately sensitive (+): diameter between 8 and 14 mm.
• Sensitive (++): diameter between 14 and 20 mm.
• Extremely sensitive (+++): diameter > 20 mm.
Actually, the essential oil of J. oxycedrus berries showed different inhibitory activities on the strains. Especially against Staphylococcus aureus ATCC11778, exerted medium activity with a diameter of 20 mm, on the other hand phoenecea oil significantly inhibited the growth of Staphylococcus aureus ATCC11778. While a significant and higher activity than phoenecea oil was found against Escherichia coli: ATCC25922 and Candidas albicans: ATCC10231 with a diameter of 30 mm and against Bacillus cereus: ATCC25923 with a diameter of 25 mm. Pseudomonas aeruginosa: ATCC10231 which is also a gram negative bacterium showed the smallest diameter of 21 mm against the oxycedar oil. The inhibitory power of the essential oils was confirmed by MIC tests. The minimum inhibitory concentrations (MIC) in µg/ml of the essential oils of J. phoenicea and J. oxycedrus obtained by the direct contact method are shown in Table 4 and 5, where they were strongly correlated with the diameters of the inhibition zones. According to the sensitivity, the lowest MIC (3.12 µg/mL) was obtained for the essential oil of J. phoenecea on the strains of Pseudomonas aeruginosa: ATCC10231 and Bacillus cereus: ATCC25923. In the case of J. oxycedrus essential oil, the MIC value was not determined against the Staphylococcus aureus strain ATCC11778, which proved to be extremely resistant.
| Strain | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.3 | 0.1 | 0 | T+ | T- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | - | MIC | + | + | + | + | + | + | + | + | + | - |
| Pseudomenas | - | - | - | - | MIC | + | + | + | + | + | + | - |
| Staph | - | - | MIC | + | + | + | + | + | + | + | + | - |
| Bacillus | - | - | - | - | MIC | + | + | + | + | + | + | - |
| Candida | - | - | MIC | + | + | + | + | + | + | + | + | - |
Table 4. MIC of J. phoenecea oil.
| Strain | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.3 | 0.1 | 0 | T+ | T- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | MIC | + | + | + | + | + | + | + | + | + | + | - |
| Pseudomenas | - | MIC | + | + | + | + | + | + | + | + | + | - |
| Staph | + | + | + | + | + | + | + | + | + | + | + | - |
| Bacillus | MIC | + | + | + | + | + | + | + | + | + | + | - |
| Candida | - | MIC | + | + | + | + | + | + | + | + | + | - |
Table 5. MIC of J. Oxycedrus oil.
Our results reveal that our plant oil collected from the Tiaret region showed a good inhibitory activity on Gram-negative bacteria such as Pseudomonas aeruginosa: ATCC10231 and Gram-positive bacteria Staphylococcus aureus: ATCC11778; on the other hand, it is weakly active on the Escherichia coli strain: ATCC25922. The bacteria most sensitive to the action of the essential oils studied in our study are the Gram-positive bacteria. According to the zones of inhibition and MIC values generated by the essential oils studied, the essential oil of J. phoenecea presents the best activity on all the strains tested. Of the two oils tested, the antimicrobial activities were recorded on all the strains are almost in agreement with those reported by (Rahhal et al., 2019) in which it was shown against Staphylococcus aureus by J. oxycedrus oil. On the other hand, according to the study of (Sela et al., 2013) revealed lower activities when compared to the activity found in our study which enhances the antibacterial effect of J. J. oxycedrus oil (< 20 mm) towards Escherichia coli, Pseudomonas aeruginosa and Candidas albicans strains. The inhibitory effect of J.phenecea oil against the strains shows significant results compared to the studies of (Rahhal et al., 2019, Mazari et al., 2010; Ennajar et al., 2009) shows no antibacterial activity against Escherichia coli. Other works such as (Abdeli, 2018) show significant results against Bacillus and Candidas albicans by a diameter of 34.67 mm and against Escherichia coli, with a similar effect to our results on Candidas albicans (Bouyahyaoui et al., 2016). These results are difficult to compare as the methods used are different. The choice of the extraction protocol, other factors such as climate, region and harvesting period of the species studied..., etc., are all factors that may influence the results. Therefore, the structural of the cell wall of (Gram+) bacteria is less complex than that of (Gram-) bacteria. This structural difference makes it less sensitive to the effects of essential oils and plant extracts (Kalemba and Kunicka, 2003). According to (Nikaido, 2003); Gram- negative bacteria have the outer membrane as an effective permeability barrier; the lipopolysaccharide, thanks to its negative surface charges prevents the diffusion of hydrophobic molecules, while Gram+ positive bacteria are less protected because the wall is formed by a peptidoglycan layer only which hinders the diffusion of molecular weights at 50 KD. The chemical composition of the essential oils studied for their antibacterial activity is dominated by the presence of hydrocarbon molecules. Many works have also confirmed that the activity of essential oils is related to most of the compounds and to a possible synergy between the components (Oussou et al., 2008; Oussou et al., 2010; Saint, 2003, Kalemba and Kunicka, 2003).
Conclusion
The essential oil of the aerial part of J. oxycedrus is mainly dominated by the hydrocarbon monoterpene class (69.2%). It should be noted that this family is characterised by the abundance of α-pinene (29.1%) and β-pinene (17.6%). The hydrocarbon sesquiterpenes (27.2%) are clearly dominated by β-copaene (19.3%). While, the J. phoenecea essential oil profile is also characterised by its richness in hydrocarbon monoterpenes (71.2%) with β-pinene (35.7%) as the majority compound. The results of this work show that the variation in the chemical composition of J. phoenecea and J. oxycedrus essential oils in the Tiaret region may be due to various factors such as: collection region, harvesting season, species and subspecies, plant parts and fruit maturity, as well as to the extraction method used, genetic background of the species or environmental factors such as altitude, climate, soils and precipitations. The microbiological study revealed that our oils possess antimicrobial properties, which can be used as natural antimicrobial agents for human and especially infectious pathologies and also as food or in medicinal formulations. In addition, the presence of major compounds such as terpenes (α-pinene, β-pinene, limonene, sabinene) in these oils, they promote it and give it this faculty to have this strong antimicrobial and antifungal activity very interesting.
References
Abdellia, W., Bahria, F., Höferlb, M., Wannerc, J., Schmidtb, E., Jirovetzb, L. (2013). Chemical composition, antimicrobial and anti-inflammatory activity of Algerian Juniperus phoenicea essential oils. Natural Product Communications, 13:223-228.
Adams, R.P., Altarejos, J., Fernandez, C., Camacho, A. (1999). The leaf essential oilsand taxonomy of Juniperus oxycedrus L. subsp. oxycedrus, subsp. badia (H. Gay) Debeaux, and subsp. macrocarpa (Sibth. Sm.) Ball. Journal of Essent Oil, Research, 11:167-172.
Adams, R.P. (2008). Junipers of the World: The genus Juniperus. (2nd Edn). Vancouver, BC, Canada: Trafford Publishing.
Google Scholarssssss
Alan, S., Glu, M., Ener, G. (2016). Composition of the essential oils of Juniperus oxycedrus L. subsp. oxycedrus growing in Turkey. Turkish Journal of Pharmaceutical Sciences, 13:300-303.
Amer, M., Wasif, M.M., Abo-Aytta, A.M. (1994). Chemical and biological evaluation of Juniperus phoenicea as a hypoglycemic agent. Journal of Agricultural Research, 21:1077-1091.
Andrews, J.M. (2001). Determination of minimum inhibitory concentrations. Journal of antimicrobial Chemotherapy, 48 :5-16.
Angioni, A., Barra, A., Russo, M.T., Coroneo, V., Dessi, S., Cabras, P. (2003). Chemical composition of the essential oils of Juniperus from ripe and berries and leaves and their antimicrobial activity. J Agricultural Food Chemistry 51:3073-3078.
Baba-Aïssa, F. (1991). Les plantes médicinales en Algérie. Diwan, Alger, p:181.
Bahri, F., Romane, A., Arjouni, Y., Harrak, R., El Alaoui, M. (2011). Chemical composition and anti-bacterial activity of the essential oil of moroccan Juniperus phoenicea. Natural Product Communications. 6(10):1515 -1518.
Bellakhder, J. (1997). La pharmacopée marocaine traditionnelle. Ibis Press, Paris, France, p:272.
Boudiba, S., Hanini, K., Selatnia, I., Saouane, A., Hioun, S., Benahmed, M. (2019). Experimental, theoretical and mathematical studies of Echiumitalicum L. extract as a corrosion inhibitor for carbon steel in acidic medium. Materials Research Express, 6:086546.
Boudjelal, A., Henchiri, C., Sari, M. (2013). Herbalists and wild medicinal plants in M'Sila (North Algeria): an ethnopharmacology survey. Journal of Ethnopharmacology, 148:395-402.
Boudy, P. (1950). Guide du forestier en Afrique du nord. Tome IV, Paris, pp:274-278.
Bouyahyaouia, A., Bahria, F., Romaneb, A., Höferlc, M., Wannerd, J., Schmidtc, E., Jirovetz, L. (2016). Antimicrobial activity and chemical analysis of the essential oil of Algerian Juniperus phoenicea. Natural Product Communication, 11:519-522.
Bouzouita, N., Kachouri, F., Ben Halima, M., Chaabouni, M. (2008). Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperus phoenicea. Journal de la Société Chimique de Tunisie, 10:119-125.
Deans, S.G., Ritchie, G. (1987). Antibacterial properties of plant essential oils. International Journal of Food Microbiology 5:165-180.
Derwich, E., Benziane, Z., Boukir, A. (2010). Chemical composition of leaf essential oil of Juniperus phoenicea and evaluation of its antibacterial activity. International Journal of Agriculture and Biology, 12:199-204.
Djabou, N., Lorenzi, V., Guinoiseau, E., Andreani, S., Giuliani, M.C., Desjobert, J.M., Muselli, A. (2013). Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against food borne or toxi-infectious pathogens. Food Control, 30: 354–63.
Dob, T., Dahmane, D., Chelghoum, C. (2006). Essential oil composition of Juniperus oxycedrus growing in Algeria. Pharmaceutical Biology, 44:1-6.
Dob, T., Dahmane, D., Chelghoum, C. (2008). Chemical composition of the essential oil of Juniperus phoenicea L. from Algeria. The Journal of Essential Oil Research, 20:15-20.
Ennajar, M., Bouajila, J., Lebrihi, A., Mathieu, F., Abderraba, M., Raies, A., Romdhane, M. (2009). Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees). Food Microbiology and Safety.
Fadel, H., Benayache, F., Chalchat, J.C., Figueredo, G., Chalard, P., Hazmoune, H., Benayache, S. (2019). Essential oil constituents of Juniperus oxycedrus L. and Cupressus sempervirens L. (Cupressaceae) growing in Aures region of Algeria. Natural Product Research, pp:1-5.
Farjon, A. (1992). The taxonomy of the multi seed junipers (Juniperus sect Sabina) in South West Asia and east Africa. Edinb Journal of Botany, 49:251-283.
Garrett, R.H., Grisham, C.M. (2000). Les transports membranaires. In Biochimie. Ed De Boeck, pp:314.
Hammer, K.A., Carson, C.F., Riley, T.V. (1999). Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology, 86:985-990.
Harhour, A., Brada, M., Fauconnier, M.L., Lognay, G. (2018). Activité antimicrobienne des huiles essentielles du Juniperus pheonicea. 1er Symposium International sur la Recherche Antimicrobienne (ISAR 2018).
Kalemba, D., Kunicka, A. (2003). Antibacterial and antifungal properties of essential oils. Current Medicinal Chemistry, 10:813-829.
Le Floc’h, E. (1983). Contribution à une étude ethnobotanique de la flore tunisienne. MES et RS, Tunis, Tunisia, p:402.
Loizzo, M., Tundis, R., Conforti, F., Saab, A., Statti, G., Menichini, F. (2007). Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chemistry, 105:572-578.
Louni, D. (1994). Les forêts algériennes. Forêt Méditerranéenne, 15:59-63.
Mann, C.M., Cox, S.D., Markham, J.L. (2000). The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributrs to its tolerance to the essential oil of Melaleuca alternifolia (Teatree oil). Letters in Applied Microbiology, 30: 294-297.
Mazari, K., Bendimerad, N., Bekhechi, C., Fernandez, X. (2010). Chemical composition and antimicrobial activity of essential oils isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. Journal of Medicinal Plants Research, 4:959-964.
Miceli, N., Marino, A., Köroğlu, A. (2020). Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus L. (Cupressaceae) taxa growing in Turkey. Natural Product Research, 34:1636-1641.
Nikaido, H. (2003). Molecular basis of bacterial outer membrane permeability revisites. Microbiology and Molecular Biology Reviews, 67:593-656.
Oussou, K.R. (2008). Etude chimique et activité biologiques des huiles essentielles desept plantes aromatiques de la pharmacopée Ivoirienne. Doctorat de l’Université de Cocody-Abidjan, p:241.
Oussou, K.R., Youlou, S., Kanko, C., Tue, B.B., Kanko, C., Boti, J.B., Ahibo, C., Casanova, J. (2010). Etude Chimique Bio-Guidée de L’huile Essentielle de Ocimum gratissimum (Lamiaceae). European Journal of Scientific Reaserch, 1:50-59.
Perez, C., Pauli, M., Bazerque, P. (1990). An antibiotic assay by agar well diffusion method. Acta Biologiae et Medicinae Experimentalis, 15:113-115.
Quezel, P., Santal, S. (1963). Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales. Paris, France, CNRS Edition, pp:34-38.
Quezel, P., Medail, F. (2003). Ecologie et biogéographie des forêtsméditerranéennes. Paris, Elsevier.
Rahhal, R., EL-Hajjouji, H., Gmouh, S., Hsaine, M., Fougrach, H., Badri, W. (2019). Chemical composition, antioxidant and antibacterial activities of the essential oils of Juniperus phoenicea, Juniperus thurifera and Juniperus oxycedrus. Mediterranean Journal of Chemistry, 9(3) : 190-198.
Ramdani, M., Lograda, T., Chalard, P., Chalchat, J.C., Figueredo, G. (2011). Chemical variability of essential oils in natural populations of Cupressus dupreziana. Natural Product Communication, 6:87-92.
Ramdani, M., Lograda, T., Silini, H., Zeraib, A., Chalard, P., Figueredo, G., Bouchaala, M., Zerrar, S. (2013). Antibacterial activity of essential oils of Juniperus phoenicea from Eastern Algeria. Journal of Applied Pharmaceutical Science, 3, 022-028.
Saint Laumer, D.J.Y., Frérot, E., Herrmann, A., (2003). Controlled release of perfumery alcohols by neighboring-group participation. Comparison of the rate constants for the alkaline hydrolysis of 2-acyl-, 2-(hydroxymethyl)-, and 2-carbamoylbenzoates; Helvetica Chimica Acta, 86:2871-2899.
Sela, F., Karapandzova, M., Stefkov, G., Cvetkovikj, I., Trajkovska-Dokikj, E., Kaftandzieva, A., Kulevanova, S. (2013). Chemical composition and antimicrobial activity of berry essential oil of Juniperus oxycedrus L. (Cupressaceae) grown wild in Republic of Macedonia. Macedonian pharmaceutical bulletin, 59:41-48.
Soltani, Y., Ali Bouzidi, M., Toumi, F., Benyamina, A. (2019). Activités antiinflammatoire et analgésique de l’extrait hydroalcoolique des baies de Juniperus phoeniceaL. Phytothérapie.
Author Info
A.H.A. Boukhaloua1,4*, M. Berrayah2, F. Bennabi1,4, A. Ayache1,3 and F. Abdeldjebar1,42Department of Environmental Sciences, Faculty of Natural and Life Sciences, University of Ibn Khaldoun, Tiaret 14000, Algeria
3Laboratory of Plant Biodiversity, Conservation and Valorizatio, Algeria
4Eco-development Laboratory for Spaces, Algeria
Citation: Boukhaloua, A.H.A., Berrayah, M., Bennabi, F., Ayache, A., Abdeldjebar, F. (2022). Antibacterial activity and identification by GC/MS of the chemical composition of essential oils of Juniperus phoenecea and Juniperus oxycedrus L. from Western Algeria: Tiaret province. Ukrainian Journal of Ecology. 12:31-39.
Received: 08-May-2022, Manuscript No. UJE-22-63119; , Pre QC No. P-63119; Editor assigned: 11-May-2022, Pre QC No. P-63119; Reviewed: 21-May-2022, QC No. Q-63119; Revised: 26-May-2022, Manuscript No. R-63119; Published: 31-May-2022, DOI: 10.15421/2022_372
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.