Research - (2022) Volume 12, Issue 5
Algal diversity study in the western Algerian coast
S. Mehiaoui1,2*, F. Nemchi2, Z. Bouzaza1, T. Farah3 and B. Bachir-Bouiadjra4Abstract
In order to identify species of seaweed on the algerian west coast, an exhaustive checklist was carried out. A total of 162 species was identified spread over three phyla: 77 species of Rhodophyceae, 48 species Pheophyceae and 37 species Chlorophyceae. Among the algae listed, six are considered invasive in the Mediterranean. The overall ratio of Rhodophyceae to Pheophyceae (R/P) is estimated at 2.19 for the entire study area.This is close to the national average estimated at 3 as well as other areas of the Mediterranean. The calculation of the Shannon-Weaver (H') and Equitability (E') diversity index identifies the ecological status of the sites which varies more or less moderately (2.53 ≤ H' ≤ 3.09; 1.38 ≤ E' ≤ 1.75) for the Ben Abdelmalek ramdan, Petit port, Bahra and El-Geulta sites, to mediocre (1.03 ≤ H' ≤1.24; 0.55 ≤ E' ≤ 0.84) for Saint-Michel, Mers El-Hadjadj, Stidia, Ouréah, Sablette, Salamander and El-Marsa. A Correspondence Analysis (CA) supported previous analysesin order to find the relation between the geographical position of the chosen sites and the algae inventoried. This analysis revealed that a floristic composition representative of healthy waters distinguishes the two sites Abdelmalek Ramdan and Bahara. This study could be a valuable tool in the field of biomonitoring and conservation biology.
https://sporbahisleri.blogaaja.fi http://sporbahisleri.parsiblog.com https://spor-bahisleri.jimdosite.com https://sporbahisleri.edublogs.org https://sporbahisleri.websites.co.in https://sporbahisleri.podia.com https://sporbahisleri7.wordpress.com https://sporbahisleri.jigsy.com https://niwn-chroiaty-mcieung.yolasite.com https://spor-bahisleri.mywebselfsite.net https://sporbahisleri.mystrikingly.com https://sporbahisleri.splashthat.com https://sporbahisleri1.webnode.com.tr https://sporbahisleri.odoo.com http://sporbahisleri.creatorlink.net http://www.geocities.ws/sporbahisleri/ https://spor-s-site.thinkific.com https://artistecard.com/sporbahisleri https://sporbahisleri.estranky.cz https://spor-bahisleri.mozellosite.com https://651be6b563e56.site123.me https://betsitesiinceleme.blogspot.com https://sporbahisleri.hashnode.dev https://sporbahislerim.wixsite.com/spor-bahisleri https://sporbahislerix.weebly.com https://sites.google.com/view/betsiteleri https://codepen.io/sporbahisleri https://sporbahisleri.bcz.com https://www.smore.com/6rsb9
Keywords
Macrophytes, Specific diversity, Inventory, Algerian west coast.
Introduction
In coastal marine ecosystems, the existence of a plant compartment plays a key role in maintaining ecological balance (primary production) and considered an indicator for the health status of marine ecosystems (Blanfuné et al., 2017; Orfanidis, 2011; Jégou, 2011; Ballesteros, et al., 2007). This compartment also plays an economic role, particularly in direct food consumption (Marfaing and Lerat, 2007; Falquet and Hurni, 2006; Conte and Payri, 2002).
Benthic macrophytes are excellent bio-indicators of the ecological status of an environment (Gibson, et al., 2000). They are involved in improving water clarity through sediment stabilization and act as ecosystem builders; they are a substrate for epiphyton, occupy a key position in the food chain and provide critical habitats for invertebrates and fish (Haury et al., 2008; Delmail, 2011).
They are also excellent indicators of water quality due to their sedentary lifestyle (Diez et al., 1999; Belsher, 1977), they incorporate the effects of long-term exposure to nutrients and/or other pollutants leading to a decrease or disappearance of the most sensitive species and their replacement by highly resistant species (Murray and Littler, 1978). Therefore, the study of macroalgal communities becomes interesting to assess changes in water quality (Fairweather, 1990).
Seaweed has scientifically proven nutritional interests since they contain many essential compounds such as dietary fiber, protein (50-70% of their dry weight in spirulina), polyunsaturated fatty acids, vitamins (F, B12, K1, B9, C, provitamin A, etc.), mineral elements (iodine, manganese, magnesium, calcium, iron, etc.) and antioxidants (polyphenols, carotenoids,...) (Marfaing and Lerat, 2007; Falquet and Hurni, 2006).
In North Africa, particularly in Algeria, knowledge of macrophytobenthos is limited to some inventory and ecology work carried out in the centre and west of the country. This concerns both algae (Ould Ahmed et al., 2013; Seridi, 2007; Seridi et al., 2007; Benhissoune, 2002; Ould Ahmed, 1994; Verlaque and Seridi, 1991; Seridi, 1990; Perret-Boudouresque and Seridi, 1989) and phanerogams (in particular Posidonia oceanica) (Mammeria, 2006; Bouhayene, 2002; Francour, 1990; Boudouresque and Meinesz, 1982).
Referring to the inventory of benthic seaweed of the Algerian coast, a total of 497 species have been identified which are divided into three systematic groups including 315 Rhodophyceae (red algae), 99 Phaeophyceae (brown algae) and 83 Chlorophyceae (green algae) (Perret-Boudouresque and Seridi, 1989). Over the past two decades, many species of exotic algae have appeared in the Mediterranean basin via maritime traffic and especially across the Suez Canal. (Verlaque and Fritayre, 1994). We can mention the expansion of invasive Caulerpa (Caulerpa taxifolia and Caulerpa racemosa var. cylindracea) in many regions of the Mediterranean including along the North African coasts. On the Algerian coasts, C. racemosa was reported for the first time in the centre by Ould Ahmed and Meinesz (2007) then in eight other localities in the same region (Lamouti et al., 2011; Seridi and Kabrane, 2010; Ould Ahmed et Meinesz, 2007) and the Algerian west coast: Mostaganem (Bachir-Bouiadjra et al., 2010) and Oran (Bentaallah and Kerfouf, 2017).
The aim of this work is to establish an inventory of macrophyte algae species and to classify the Algerian west coast in relation to all Algerian and Mediterranean coast in terms of algal biodiversity.
Materials and Methods
Sampling stations
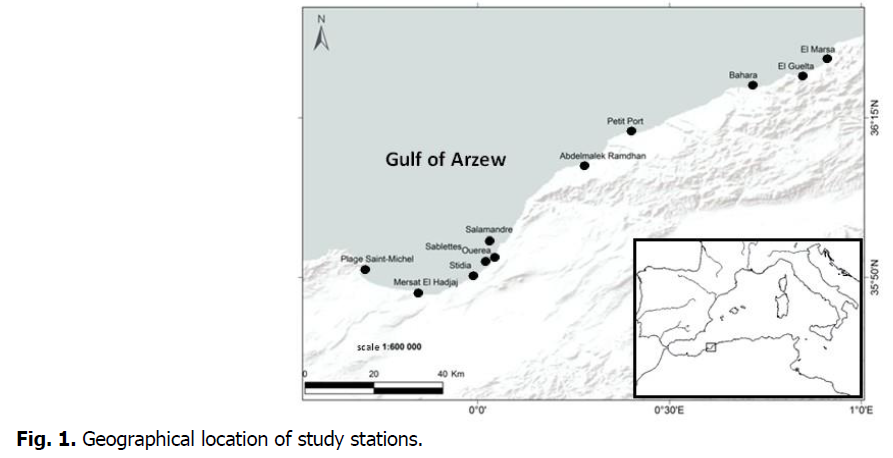
A sampling extending over eleven stations on the Algerian west coast was carried out (Fig. 1, Table 1).
Fig 1: Geographical location of study stations.
| Stations | Abbreviations | Geographic Coordinates |
|---|---|---|
| Saint-Michel | SM | 35°51’5’’N; 0°17‘40”W |
| Mers El Hadjadj | MH | 35°47’27”N; 0°09’22”W |
| Stidia | ST | 35°50’05”N; 0°00’39”W |
| Ouréah | OR | 35°52’26”N; 0°01’17”E |
| Sablette | SB | 35°53’02”N; 0°02’34”E |
| Salamandre | SL | 35°55’42”N; 0°01’52”E |
| Abdmalek Ramdan | AR | 36°07’14”N; 0°16’35”E |
| Petit port | PP | 36°12’40”N; 0°24’01”E |
| Bahara | BA | 36°19’53”N; 0°43’06”E |
| El-Guelta | EG | 36°21’17”N; 0°50’44”E |
| El-Marsa | EM | 36°23’56”N; 0°54’41”E |
Table 1. Sampling stations.
Collection and identification of seaweed
The stations were visited monthly over a period of two years between December 2018 and December 2020. The inventory established was carried out on the basis of samples taken in diving with autonomous diving suit between 0 and 40 m deep using a PVC quadrat of (20 × 50 cm). For each station we prospected a coastal linear of 25 to 50 m in length and 5 to 10 m wide taking care to recover the entire thallus and the most varieties Possible.Individuals have been carefully taken with their base, which is often a fundamental character of recognition and identification. Species identification was carried out using identification keys.
Analytical parameters
Quantitative and qualitative analyses of vegetation were carried out using terrestrial phytosociological methods adapted to the marine environment such as Shannon Diversity Index (H’) (Shannon, 1948) and equitability (E’) (Scammacca et al., 1993; Ribera et al., 1992).
Multivariate analysis
In order to assess a possible relationship between sampling stations' geographical position and the algae inventoried in each region, a Correspondence Analysis (CA) was carried out on the specific diversity of sampling sites using the R Studio program.
Results
Identification of seaweed
The analysis of the harvests carried out over the entire study area allowed us to determine a total of 162 species divided into six classes (Bangiophyceae, Floridaophyceae, Phaeophyceae, Chlorophyceae, Bryopsidophyceae, Compsogonophyceae) (Fig. 2; Table 2).
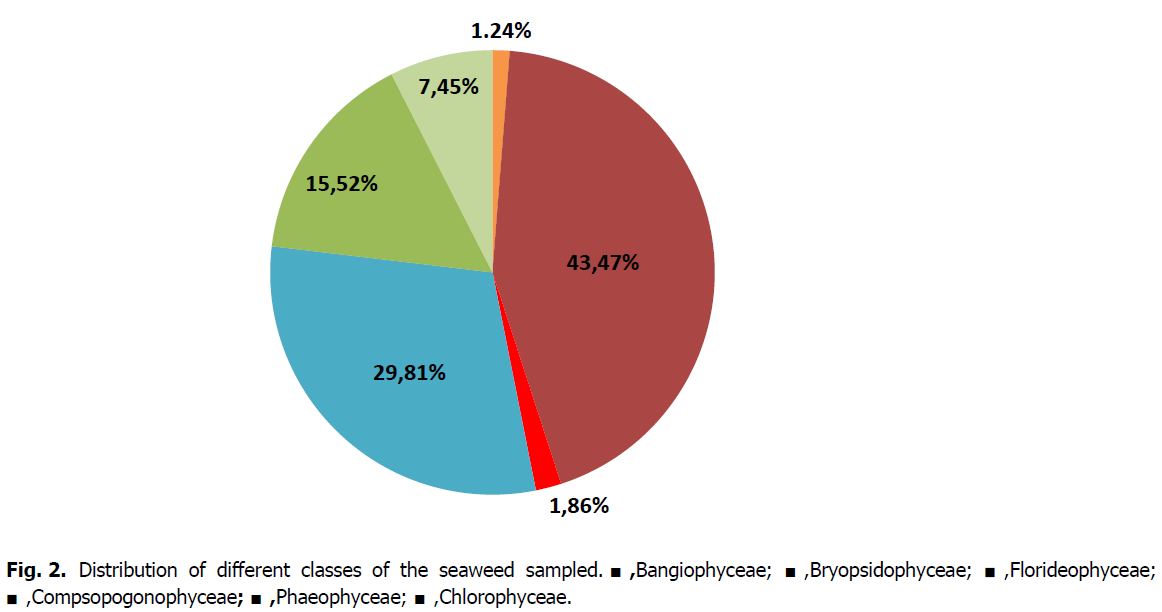
Fig 2: Distribution of different classes of the seaweed sampled. ■,Bangiophyceae; ■,Bryopsidophyceae; ■,Florideophyceae; ■,Compsopogonophyceae; ■,Phaeophyceae; ■,Chlorophyceae.
| Rhodophyceae (n=77) | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Stations | ||||||||||||||||||||||||||||||||||
| SM | MH | ST | OR | BA | SL | AR | PP | BA | EG | EM | |||||||||||||||||||||||||
| Acrothamnion preissii | + | - | + | + | - | + | + | + | - | - | + | ||||||||||||||||||||||||
| Acrochaetium hamelii | - | - | + | - | - | + | + | + | + | - | - | ||||||||||||||||||||||||
| Acrochaetium caesareae | - | - | + | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Acrochaetium cheminii | + | + | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Acrochaetium crassipes | + | - | - | - | - | + | - | + | + | - | - | ||||||||||||||||||||||||
| Amphiroa beauvoisii | + | + | - | - | - | + | - | + | - | + | + | ||||||||||||||||||||||||
| Amphiroa rigida | - | - | - | + | + | - | + | + | + | - | - | ||||||||||||||||||||||||
| Anotrichium secundum | - | - | - | - | + | - | + | - | + | - | - | ||||||||||||||||||||||||
| Antithamnion amphigeneum | - | + | - | + | + | - | - | + | + | - | - | ||||||||||||||||||||||||
| Aparagpsis armata | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Asparagpsis taxiformis | - | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Bliding Chylocladiaverticillata | - | - | - | - | - | - | + | - | + | + | + | ||||||||||||||||||||||||
| Bonnemaisonia asparagoides | + | - | - | - | + | - | - | + | - | + | + | ||||||||||||||||||||||||
| Bonnemaisonia hamifera | + | - | - | - | - | - | - | + | - | - | + | ||||||||||||||||||||||||
| Centroceras clavulatum | - | - | - | + | + | - | - | + | + | + | - | ||||||||||||||||||||||||
| Ceramium codii | + | + | - | - | - | + | - | - | + | + | - | ||||||||||||||||||||||||
| Ceramium diaphanum | - | - | - | - | - | + | + | + | + | + | - | ||||||||||||||||||||||||
| Chondracanthus acicularis | + | - | + | + | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Chondracanthus teedei | + | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Chondria capillaris | - | + | - | - | + | - | + | + | - | - | - | ||||||||||||||||||||||||
| Chondria coerulescens | - | - | - | - | + | + | + | + | - | + | - | ||||||||||||||||||||||||
| Chondria dasyphylla | + | - | + | - | - | + | - | + | - | + | - | ||||||||||||||||||||||||
| Chondria mairei | + | - | + | - | - | + | - | + | - | - | + | ||||||||||||||||||||||||
| Colaconema daviesii | - | - | + | + | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Colaconema leptonema | - | - | + | + | - | - | - | + | - | + | + | ||||||||||||||||||||||||
| Corallina officinalis | - | + | + | + | - | + | + | + | - | + | + | ||||||||||||||||||||||||
| Corallophilacinnabarina | - | + | + | + | - | - | + | - | - | + | - | ||||||||||||||||||||||||
| Dasya rigidula | - | - | + | - | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Digenea simplex | - | - | + | + | - | - | - | + | + | - | + | ||||||||||||||||||||||||
| Ellisolandia elongata | + | + | + | - | - | + | + | + | + | + | + | ||||||||||||||||||||||||
| Erythrotrichiabertholdii | - | + | - | - | - | - | + | + | - | - | + | ||||||||||||||||||||||||
| Erythrotrichiacarnea | - | + | - | + | - | + | - | + | - | - | - | ||||||||||||||||||||||||
| Gastroclonium clavatum | + | - | - | - | - | + | - | + | - | + | + | ||||||||||||||||||||||||
| Gelidium corneum | + | + | + | - | - | + | - | + | - | - | - | ||||||||||||||||||||||||
| Gelidium crinale | - | - | + | - | - | + | - | + | - | - | - | ||||||||||||||||||||||||
| Gelidium lubrica | - | + | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Gelidium spinosum | + | - | + | - | - | + | - | - | - | + | + | ||||||||||||||||||||||||
| Gracilaria bursa-pastoris | - | + | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Gracilariopsislongissima | - | + | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Grateloupiafilicina | - | + | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Halopithys incurva | + | - | - | - | - | - | - | - | + | + | - | ||||||||||||||||||||||||
| Herposiphonia seconda | - | - | - | - | - | + | - | + | + | - | - | ||||||||||||||||||||||||
| Heterosiphonia crispella | - | - | - | - | - | + | - | + | - | - | - | ||||||||||||||||||||||||
| Huismaniella ramellosa | - | + | - | - | - | + | - | + | + | - | - | ||||||||||||||||||||||||
| Hypnea musciformis | - | + | - | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Jania longifurca | + | - | - | - | - | + | - | + | - | - | + | ||||||||||||||||||||||||
| Jania rubens | + | + | + | - | - | + | + | + | + | + | + | ||||||||||||||||||||||||
| Laurencia microcladia | - | - | + | - | - | - | - | + | + | - | - | ||||||||||||||||||||||||
| Laurencia obtusa | - | - | + | - | - | - | - | + | + | - | - | ||||||||||||||||||||||||
| Leptosiphonia fibrillosa | - | - | + | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Liagora viscida | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Lithophyllum incrustans | + | - | + | - | - | + | - | + | - | + | + | ||||||||||||||||||||||||
| Lithothamnio nvalens | + | - | - | - | - | - | - | + | + | - | - | ||||||||||||||||||||||||
| Lomentaria articulata | + | - | - | - | - | - | + | + | - | - | + | ||||||||||||||||||||||||
| Mesophyllum alternans | + | - | + | - | - | - | - | - | - | + | - | ||||||||||||||||||||||||
| Mesophyllum lichenoides | + | + | + | - | - | + | + | + | - | + | + | ||||||||||||||||||||||||
| Nemalionelm inthoides | + | - | + | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Neosiphonia sertularioides | - | - | + | - | + | - | - | + | - | - | - | ||||||||||||||||||||||||
| Palisada perforata | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Peyssonnelia heteromorpha | - | - | - | - | - | - | + | - | - | - | - | ||||||||||||||||||||||||
| Peyssonnelia rosa-marina | - | - | + | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Peyssonnelia rubra | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Peyssonnelia squamaria | - | - | + | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Plocamium cartilagineum | - | - | + | - | - | + | - | + | - | + | - | ||||||||||||||||||||||||
| Polysiphonia deusta | - | - | + | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Porphyra umbilicalis | - | - | + | - | + | - | - | + | - | - | - | ||||||||||||||||||||||||
| Pterocladiella capillacea | + | + | + | - | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Pyropia leucosticta | + | - | + | - | - | - | - | + | + | + | - | ||||||||||||||||||||||||
| Rissoella verruculosa | - | - | + | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Sahlingia subintegra | - | - | - | - | - | - | + | - | - | - | - | ||||||||||||||||||||||||
| Schotteranicaeensis | - | - | - | - | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Sphaerococcus coronopifolius | + | - | + | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Spyridia filamentosa | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Taeniom ananum | - | - | + | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Titanodermapustulatum | - | - | - | - | - | - | - | + | - | + | - | ||||||||||||||||||||||||
| Vertebratafruticulosa | - | + | + | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Vertebratafurcellata | - | - | + | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Pheophyceae (n=48) | |||||||||||||||||||||||||||||||||||
| Species | Stations | ||||||||||||||||||||||||||||||||||
| SM | MH | ST | OR | SB | SL | AR | PP | BA | EG | EM | |||||||||||||||||||||||||
| Arthrocladiavillosa | + | + | + | - | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Asperococcusbullosus | + | + | + | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Cladosiphon mediterranéen | - | - | - | + | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Cladostephusspongiosus f. verticillatus | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Colpomeniaperegrina | - | + | + | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Colpomeniasinuosa | - | - | - | - | - | + | + | + | + | - | - | ||||||||||||||||||||||||
| Cutleriaadspersa | - | - | - | + | - | - | + | + | + | - | - | ||||||||||||||||||||||||
| Cutleriachilosa | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Cystoseiraamentacea var. stricta | - | + | + | - | + | - | + | + | + | + | - | ||||||||||||||||||||||||
| Cystoseirabrachycarpa | - | - | - | - | - | + | + | + | + | - | - | ||||||||||||||||||||||||
| Cystoseira compressa | - | - | - | - | - | - | + | + | + | + | + | ||||||||||||||||||||||||
| Cystoseiracrinita | - | - | - | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Cystoseirafoeniculacea | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Cystoseiramediterranea | - | - | - | - | - | - | + | + | + | - | - | ||||||||||||||||||||||||
| Cystoseirasedoides | - | - | - | - | - | - | - | - | + | - | - | ||||||||||||||||||||||||
| Cystoseiratamariscifolia | - | - | - | - | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Cystoseirazosteroides | - | - | - | + | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Dictyopterisdivaricata | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Dictyopterispolypodioides | - | + | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Dictyotadichotomaf.dichotoma | - | - | - | - | + | - | + | + | - | - | - | ||||||||||||||||||||||||
| Dictyotafasciola | - | - | + | - | - | - | - | - | + | + | - | ||||||||||||||||||||||||
| Dictyotaspiralis | - | + | - | - | + | - | + | + | + | + | - | ||||||||||||||||||||||||
| Ectocarpuscommensalis | + | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Ectocarpusfasciculatus | + | - | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Ectocarpussiliculosus | - | - | - | - | - | - | + | + | - | + | + | ||||||||||||||||||||||||
| Feldmanniaglobifera | - | - | - | - | - | - | - | + | + | - | - | ||||||||||||||||||||||||
| Feldmanniamitchelliae | - | - | - | - | - | - | + | - | + | - | - | ||||||||||||||||||||||||
| Feldmannia simplex | - | - | - | - | - | - | - | + | + | + | - | ||||||||||||||||||||||||
| Halopterisfilicina | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Halopterisscoparia | - | - | - | - | - | - | - | - | - | - | + | ||||||||||||||||||||||||
| Hincksiasandriana | + | - | - | - | - | - | - | - | - | - | + | ||||||||||||||||||||||||
| Hydroclathrusclathratus | - | - | - | + | - | - | - | + | - | - | + | ||||||||||||||||||||||||
| Laminariaochroleuca | - | - | - | + | - | - | + | + | + | + | - | ||||||||||||||||||||||||
| Laminariarodriguezii | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Leathesia marina | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Myriactulagracilariae | + | - | - | - | - | + | - | - | - | - | - | ||||||||||||||||||||||||
| Myriactularigida | + | - | - | - | - | + | - | - | - | - | - | ||||||||||||||||||||||||
| Myriactularivulariae | + | - | - | - | - | + | - | - | - | - | - | ||||||||||||||||||||||||
| Padinapavonica | + | + | + | - | + | - | + | + | + | + | - | ||||||||||||||||||||||||
| Phacelariacirrosa | - | - | - | + | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Ralfsiaverrucosa | - | - | - | - | - | + | + | + | + | + | + | ||||||||||||||||||||||||
| Sargassumacinarium | - | - | - | - | + | - | - | + | - | - | - | ||||||||||||||||||||||||
| Sargassumvulgare | - | + | + | - | + | - | - | - | + | + | + | ||||||||||||||||||||||||
| Sphacelariaplumula | - | - | - | - | - | + | + | + | - | - | - | ||||||||||||||||||||||||
| Taoniaatomaria | - | - | - | - | - | - | - | + | + | + | + | ||||||||||||||||||||||||
| Treptacanthaalgeriensis | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Treptacanthaballesterosii | - | - | - | + | - | + | + | - | + | - | + | ||||||||||||||||||||||||
| Treptacanthabarbata | - | + | + | - | + | - | + | - | + | + | + | ||||||||||||||||||||||||
| Chlorophyceae (n=37) | |||||||||||||||||||||||||||||||||||
| Species | Stations | ||||||||||||||||||||||||||||||||||
| SM | MH | ST | OR | SB | SL | AR | PP | BA | EG | EM | |||||||||||||||||||||||||
| Acetabularia acetabulum | + | + | + | - | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| Anadyomene stellata | - | - | - | - | - | + | + | + | - | - | + | ||||||||||||||||||||||||
| Blidingia marginata | - | - | - | - | - | + | - | - | + | + | + | ||||||||||||||||||||||||
| Bourse codium | + | + | + | - | + | + | + | - | + | - | + | ||||||||||||||||||||||||
| Bryopsis duplex | - | - | + | - | + | + | - | + | - | + | + | ||||||||||||||||||||||||
| Bryopsis hypnoides | + | - | + | - | - | + | + | + | - | + | + | ||||||||||||||||||||||||
| Bryopsis muscosa | + | - | + | - | - | + | - | + | - | + | |||||||||||||||||||||||||
| Bryopsis plumosa | + | + | + | - | - | + | - | + | - | + | + | ||||||||||||||||||||||||
| Bryopsis secunda | + | - | + | - | - | + | - | - | + | - | + | ||||||||||||||||||||||||
| Caulerpa prolifera | - | - | + | - | - | + | + | + | + | - | - | ||||||||||||||||||||||||
| Caulerpa racemosa var. cylindracea | - | - | + | - | - | + | + | + | + | - | - | ||||||||||||||||||||||||
| Chaetomorpha aerea | + | + | - | - | - | + | - | - | - | - | - | ||||||||||||||||||||||||
| Chaetomorpha capillaris | + | + | - | - | - | + | - | - | + | - | - | ||||||||||||||||||||||||
| Chaetomorpha linum | + | + | - | + | - | - | - | - | - | - | + | ||||||||||||||||||||||||
| Cladophora coelothrix | - | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Cladophora aetevirens | - | - | + | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Cladophora prolifera | - | - | + | - | - | - | + | - | + | - | - | ||||||||||||||||||||||||
| Cladophora rupestris | + | - | + | - | - | - | + | - | + | - | + | ||||||||||||||||||||||||
| Cladophoropsis membranacea | + | + | - | - | - | - | - | + | - | - | + | ||||||||||||||||||||||||
| Codium effusum | - | - | - | - | - | - | - | + | - | - | - | ||||||||||||||||||||||||
| Codium fragile | + | + | + | + | + | + | - | - | - | - | + | ||||||||||||||||||||||||
| Codium tomentosum | + | + | + | + | + | + | - | - | - | + | - | ||||||||||||||||||||||||
| Flabellia petiolata | + | - | - | - | - | - | + | + | - | - | - | ||||||||||||||||||||||||
| Lychete pellucide | + | - | - | - | - | - | + | + | - | - | + | ||||||||||||||||||||||||
| Palmophyllum crassum | - | - | - | - | - | - | + | - | + | + | - | ||||||||||||||||||||||||
| Rhizoclonium riparium | - | - | - | + | - | - | - | - | - | - | + | ||||||||||||||||||||||||
| Ulva clathrata | - | - | - | - | - | + | - | - | - | - | + | ||||||||||||||||||||||||
| Ulva compressa | + | + | - | + | - | + | - | - | - | - | - | ||||||||||||||||||||||||
| Ulva elegans | - | + | - | - | - | + | - | - | - | - | + | ||||||||||||||||||||||||
| Ulva fasciata | - | - | - | - | - | - | + | + | - | - | + | ||||||||||||||||||||||||
| Ulva intestinalis | + | - | + | - | + | + | - | - | + | + | + | ||||||||||||||||||||||||
| Ulva lactuca f. rigida | - | - | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Ulva lactuca | + | + | + | + | + | + | + | + | - | + | + | ||||||||||||||||||||||||
| Ulva linza | - | + | - | - | - | + | - | - | - | - | + | ||||||||||||||||||||||||
| Ulva prolifera | + | + | - | - | - | - | - | + | - | - | + | ||||||||||||||||||||||||
| Ulvaria obscura | - | - | + | - | - | + | + | - | + | - | + | ||||||||||||||||||||||||
| Valonia macrophysa | - | + | + | - | + | - | - | + | - | + | + | ||||||||||||||||||||||||
Table 2. Inventory of algae species sampled. (+) presence/(-) absence.
The largest number of algae species was recorded in the Petit Port site (120 species) followed by Abdelmalek ramdhan (70 species) and Bahara (60 species). However, the lowest specific wealth is recorded at the Sablette station (24 species) (Fig. 3). The identified species have been previously observed on the Algerian coast (Perret-Boudouresque and Seridi, 1989).
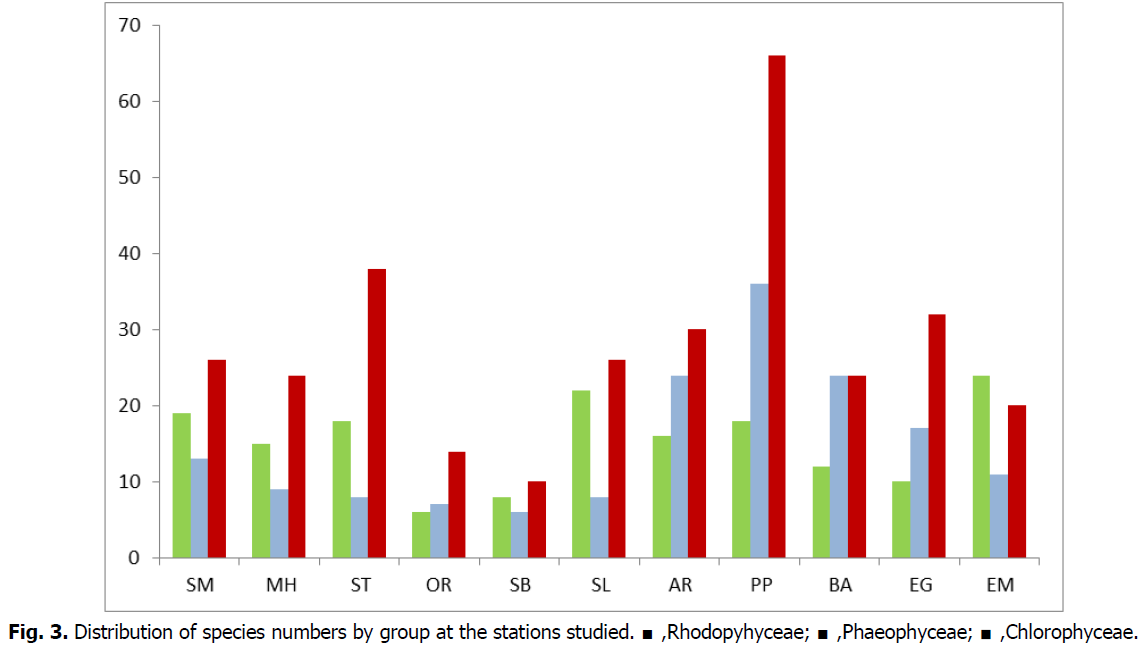
Fig 3: Distribution of species numbers by group at the stations studied. ■,Rhodopyhyceae; ■,Phaeophyceae; ■,Chlorophyceae.
The Rhodophyceae are best represented by a strong dominance of Ellisolandia elongata and Corallina officinalis which are located at the upper horizon of the infralittoral stage between 0.5 and 10 m. In addition, Asparagopsisarmata, A.taxiformis, Janiarubens and Mesophyllum lichenoides dominate the depths with a covering that reaches 60%. Phaephyceae are also well represented in Petit Port, Abdmalekramadane and Bahara with a dominance of Cystoseires: Cystoseira stricta as well as Colpomenia sinuosa, Dictyota dichotoma, Dictyota spiralis and Padina pavonica. Cholorophyceae represent a low value of 22% at the level of the stations surveyed compared to other groups. This group is dominated by Acetabularia acetabulum, Chaetomorpha aerea, Bourse codium, Ulva compressa, Ulva intestinalis and Ulva sp.
Floristic composition/total covering
The floristic composition of our surveys varies according to the stations and the observation periods, the average number of species per survey, for the entire study area the maximum is perceived in March, spring season, which is a period of specific algal diversification Ballesteros (1992), with 77 species per survey then falls in November (autumn period) to reach 54 species then a slight increase in June (summer season) with 66 Taxa (Fig. 4).
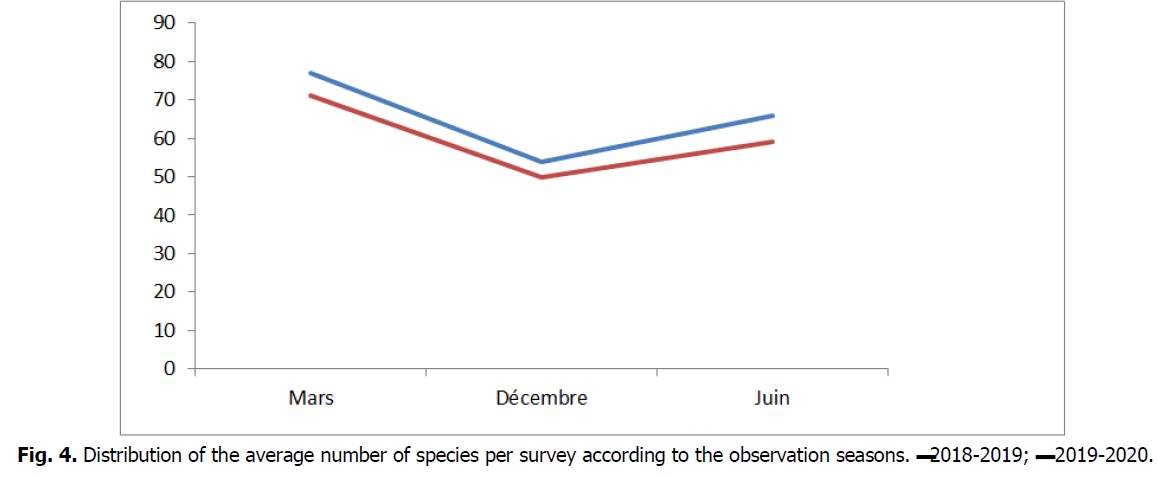
Fig 4: Distribution of the average number of species per survey according to the observation seasons. ▬2018-2019; ▬ 2019-2020.
R/P Ratio
The overall ratio of Rhodophyceae to Pheophyceae “R/P” is estimated to 2.19 for the overall study area. Bahara station has the lowest value (1) while Stidia station has the highest value (4.75) (Table 3).
| Stations | R/P |
|---|---|
| Saint-Michel | 2 |
| Mers El Hadjadj | 2.66 |
| Stidia | 4.75 |
| Ouréah | 2 |
| Sablette | 1.66 |
| Salamandre | 3.25 |
| Abdmalek Ramdan | 1.25 |
| Petit port | 1.83 |
| Bahara | 1 |
| El-Guelta | 1.88 |
| El-Marsa | 1.81 |
Table 3. R/P ratio of the different observation stations in the study area.
The diversity index of Shannon-Weaver (H') and equitability (E)
The diversity estimated using the Shannon-Weaver index (H') as well as the equitability index (E’) for all observation stations show that the least wealthy stations are (Saint-Michel, Mers el-Hadjadj, Stidia, Ouréah, Sablette, Salamandre and El-Marsa) (Fig. 5) with low values ranging from 1.03 to 1.24 for diversity (H'), faithfully followed by a equitability index (E’) oscillating between 0.55 to 0.84.These indices reflect algal stands in imbalance (Belsher andBoudouresque,1976). However, the remaining stations are more or less stable and in equilibrium(2.53 ≤ H' ≤ 3.09; 1.38 ≤ E' ≤ 1.75).
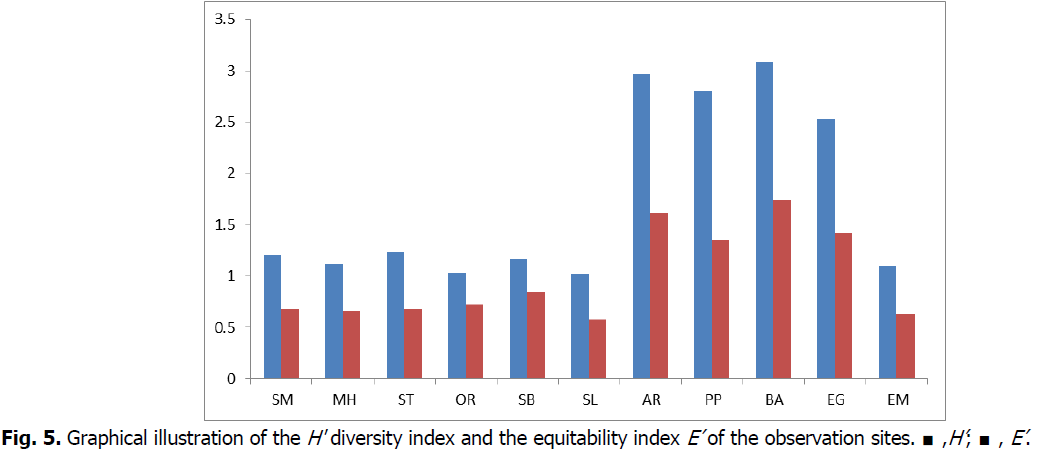
Fig 5: Graphical illustration of the H' diversity index and the equitability index E’ of the observation sites. ■,H’; ■, E’.
Multivariate analysis
The Most of the total variance is represented on the first axis (Dim 1) with 72.61% of information while the second axis (Dim 2) represents 27.39% of information (Fig. 6). The Chlorophyceae are well projected on the positive part of Dim 1 and more or less well projected on the positive part of Dim 2 and are associated with the stations El Marsa and Salamandre in particular and to a lesser extent the stations Sablettes and Saint Michel. In addition, the Phaeophyceae are well represented on the negative part of Dim 1 and more or less well represented on the positive part of Dim 2 and are associated with the Bahara and Abdelmalek Ramdan stations. As for the Rodophyceae, they are very well projected on the negative part of Dim 2 and are associated with the stations of Ouréah, El Guelta, Petit Port and especially Stidia.
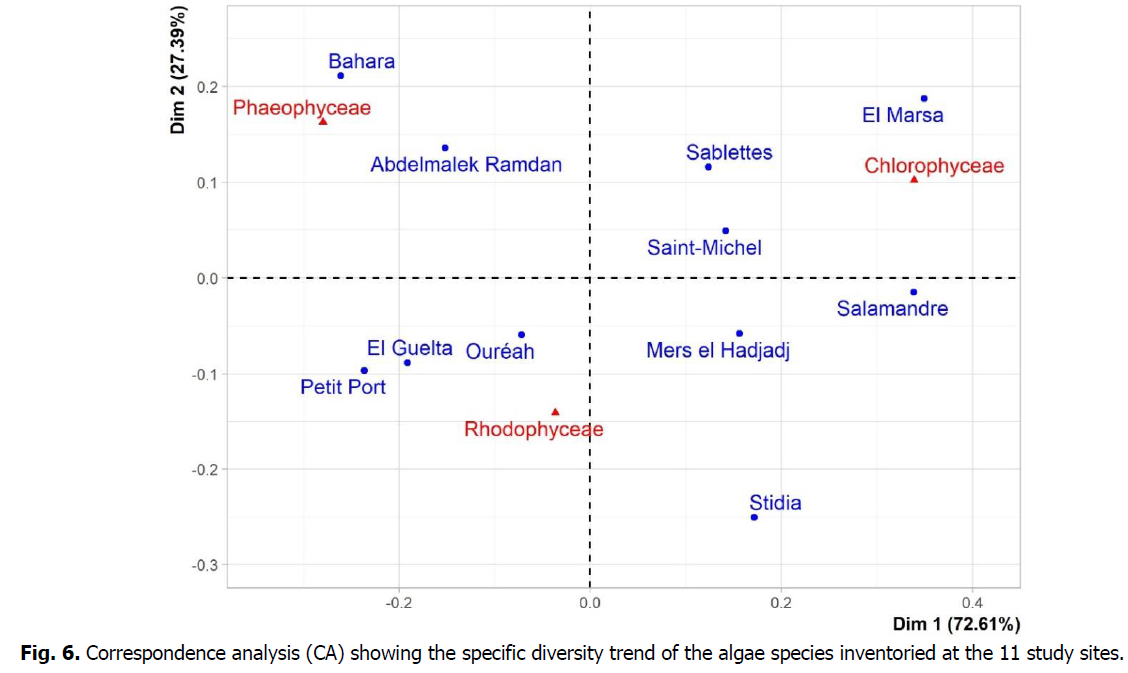
Fig 6: Correspondence analysis (CA) showing the specific diversity trend of the algae species inventoried at the 11 study sites.
Introduced/invasive algal species
Among the inventoried algae,sixare invasive: Acrothamnion preissii, Asparagopsis armata, Asparagopsis taxiformis, Caulerpa racemosa var. cylindracea, Codium fragile, Codium tomentosum. These species represent 3.70% of the total number of sampled seaweed.
Discussion
This study is an approach to the knowledge of the macrophytobenthos of the Algerian west coasts. We draw up a qualitative inventory of algae from the infralittoral floor in order to complete the database of the Algerian marine flora. We could identify 162 species divided into 3phyla: 77 species of Rhodophyceae, 48 species Pheophyceae and 37 species Chlorophyceae. There is more or less a similarity in the hierarchy of dominances of the systematic groups between the results of the present study and that of Bachir-Bouiadjra et al., (2010) and this in descending order with Rhodophyceae in the lead, followed by Phaeophyceae thenChlorophyceae.This represents a rate of 32.79% of the total number of 494 species inventoried nationally (Ould Ahmed, 1994; Seridi, 1990; Perret-Boudouresque and Serridi, 1989). Among the listed algae, six are invasive in the Mediterranean: Acrothamnion preissii, Asparagopsis armata, Asparagopsis taxiformis, Caulerpa racemosa var. cylindracea, Codium fragile, Codium tomentosum.
The dominance of Rhodophyceae compared to other taxonomic groups correlates with the Mediterranean algal flora in general. Indeed, the globalR/P ratio obtained is estimated to 2.19 for the study area which is slightly lower than the Algerian average estimated to3 (Seridi, 2004).However, the global R/P ratio obtained is close to that reported by Kazzaz (1989) (2.7 for Cabo-Negro, Tetouan-Morocco), Conde (1984) (2.41 for Malaga-Spain), Soto and Conde, (1993) (2.45 for Alboran Island) as well as Riadi (2000)(2.4for the Area of the Strait of Gibraltar). This result of R/P ratio reflects the presence in Algeria of a marine algal flora with temperate affinity of the Mediterranean type (Ould Ahmed et al., 2013). This ratio increases steadily from the cold seas of Northern Europe to the warm regions of the tropical Atlantic. It is close to 3 in the western Mediterranean, lessthan 3 in the eastern Mediterranean, 2.42 in the Adriatic (Giaccone, 1978), 2.5 in Greece and 2in Turkey (Boudouresque and Perret-Boudouresque, 1979).
The diversity estimated using the Shannon-Weaver index (H’) as well as the Equitability index (E’) for all the observation stations show that the least wealthy stations are Saint-Michel, Mers El-Hadjadj, Stidia, Ouréah, Sablette, Salamandre and El-Marsa with low values ofH’ and E’ (1.03 ≤ H ’≤ 1.24; 0.55 ≤ E’ ≤ 0.84) (Fig. 5).These indices reflect algal populations in disequilibrium (Belsher and Boudouresque, 1976). Indeed, these seaweeds are heavily affected by direct industrial, domestic and urban discharges of water without treatment, thus generating increasing pollution, inevitably affecting the algal community in its diversity (personal observation).However, the anthropogenic action characterized by urban discharges could also be the reason for the loss of algal diversity. Indeed, these stations are characterized by species of the genus Ulva such as Ulva institinalis, Ulva rigida and Ulva compressa (Table 2) which tolerate eutrophication and are known to be nitrophilic nitrogen-accumulating species (Arévalo et al., 2007; Hoffmann and Parson, 1988).
On the other hand, the stations Abelmalek Ramdane, Petit Port, Bahara and El-Geulta are relatively spared by pollution with slightly higher indices of diversity and equitability (2.53 ≤ H' ≤ 3.09; 1.38 ≤ E' ≤ 1.75) (Fig. 5). This indicates populations well differentiated, stable and in equilibrium (Chaabane, 2019; Verlaque et al., 1977) particularly for the Bahara population, Petit port and Abdmalek Ramdane (Fig. 5). The sites of Bahara and Abdelmalek Ramdan have an important floristic composition with the highest values of H’ (3.09; 2.95) and E’ (1.75; 1.6) respectively (Fig. 5). The species that characterize these two stations are algae of Pheophyceae phyla such as Cystosera sticta and Cystoseira compressa that are enormously sensitive to anthropogenic pressure and the slightest variation in the marine environment. For this purpose, these algae could be considered as bioindicators for a healthy environment. Indeed, both Bahara and Abdelmalek Ramdan are areas characterized by high hydrodynamics, a non-vertical substrate and good lighting testifies to a good environmental situation (Belmokhtar, 2012).
Moreover, the Correspondence Analysis (CA) in Fig. 6 indicates that the two sites Bahara and Abdelmalek Ramdanare diversified witha floristic composition representative of clean waters, whereas Saint-Michel, Stidia, Salamandre, Sablette and El-Marsa populations seem to be strongly disturbed by anthropogenic pollution (port areas and coastal discharges of wastewater) (Personal observation). Whereas, the sites Saint-Michel, Salamandre and El-Marsa are harbours and the rest of the stations (Saint-Michel, El-Marsa, Salamandre, Sablette, Mers el Hadjadj and Stidia) are located just near relatively polluted harbours. This is confirmed by their rarity in species sensitive to pollution as algae from Cystoseire group (Table 2) whilewe can mention the presence of pollution indicator species of the genus Enteromorpha, Chaetomorpha and Ulva (Table 2) (Rodriguez-Prieto and Polo, 1996; Ballesteros et al., 1984; Golubic, 1970).
The presence of the invasive species Caulerpa racemosa var. cylindracea (Table 2), which tends to colonize disturbed ecosystems, could explain the reduction in native algal flora (Klein andVerlaque, 2008; Klein 2007; Piazzi and Ceccherelli, 2006). Although other factors such as pollution of inorganic chemicals, increased turbidity levels, overgrazing and climate change have been suggested as other possible causes (Cormaci and Furnari, 1999). This means a highly disturbed environment that requires follow-up in the future.
References
Arévalo, R., Pinedo, S., Ballesteros, E. (2007). Changes in the composition and structure of mediterranean rocky-shore communities following a gradient of nutrient enrichment: descriptive study and test of proposed methods to assess water quality regarding macroalgae. Marine Pollution Bulletin, 55:104-113.
Bachir-Bouiadjra, B., Taleb, M.Z., Marouf, A., Benkada, M.Y., Riadi, H. (2010). First record of the invasive alga Caulerpa racemosa (Caulerpales, Chlorophyta) in the Gulf of Arzew (western Algeria). Aquatic Invasions, 5:97-101.
Ballesteros, E., Pérez, M., Zabala, M. (1984). Aproximación al conocimiento de las comunidades algales de la zona infralitoral superior en la costa catalana. Collectanea Botanica, 15:69-100.
Ballesteros, E. (1992). Contribució al coneixement algològic de la Mediterrània espanyola, IX. Espècies interessants de les illes Balears. Folia Botanica Miscelanea, 8:77-102.
Ballesteros, E., Toras, X., Pinedo, S., Garcia, M., Mangialajo, L., De Tores, M. (2007). A New methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water framework directive. Marine Pollution Bulletin, 55:172-180.
Bellan, G., Bellan-Santini, D. (1972). Influence de la pollution sur les peuplements marins de la re´gion de Marseille. In: Ruivo, M. (Ed.), Marine Pollution and Sea Life. Fishing News Ltd. Survey, pp:396-401.
Belmokhtar, M. (2012). Cystoseira amentacea var. stricta: indicateur de la qualité des eaux côtières de l’ouest Algérien. Master Thesis in Environnemental Sciences. University of Oran.
Belsher, T., Boudouresque, C.F. (1976). L’impact de la pollution sur la fraction algale des peuplements benthiques de la Méditerranée. In : Atti tavola rodenta international (la biologia marina per la difesa e per la productivita del mare), Livorno, pp:215-260.
Belsher, T. (1977). Analyse des répercussions des pollutions urbaines sur le macrophytobenthos de Méditerranée (Marseille, Port-Vendres, Port-Cros). PhD Thesis in Oceanology. Université of Aix-Marseille II.
Benhissoune, S. (2002). A checklist of the seaweeds of the mediterranean and atlantic coasts of Morocco. III. Rhodophyceae (Excluding Ceramiales). Botanica Marina, 45:391-412.
Bentaallah, M.E.A., Kerfouf, A. (2007). Prolifération de l'algue Caulerpa racemosa dans les écosystèmes littoraux de l'Algérie: état des lieux et des connaissances. Geographie Physique et Environnement, 7:157-164.
Blanfuné, A., Thibaut, T., Boudouresque, C.F., Macic, V., Markovic, L., Palomba, L., Verlaque, M., Boissery, P. (2017). The CARLIT method for the assessment of the ecological quality of European Mediterranean waters: Relevance, robustness and possible improvements. Ecological Indicators, 72:249-259.
Boudouresque, C.F., Perret-Boudouresque, M. (1979). Dénombrement des algues benthiques et rapport R/P le long des côtes françaises de la Méditerranée. Rapports et Proces-Verbaux des Reunions-Commission Internationale pour l'Exploration Scientifique de la Mer Mediterranee, 4:25-26.
Boudouresque, C.F., Meinesz, A. (1982). Découverte de l'herbier de Posidonie. Parc national de Port-Cros, 4:86.
Bouhayene, S. (2002). Contribution à la connaissance des herbiers à Posidonia oceanica dans la baie d’Annaba (est algérien). Master Thesis in Marine Sciences. University of Annaba.
Chaabane, F., Graf, A., Jequier, L., Coste, A.T. (2019). Review on antifungal resistance mechanisms in the emerging pathogen candida auris. Frontiers in Microbiology, 10:2788.
Conde, B.F. (1984). Species diversity: Concept, measurement, and response to clearcutting and site-preparation. Forest Ecology and Management, 8:11-22.
Conte, B.F., Payri, C. (2002). La consommation des algues en Polynésie française: Premiers résultats d'une enquête. Journal de la Société des Océanistes, pp:165-172.
Cormaci, M., Furnari, G. (1999). Changes of the benthic algal flora of the tremiti islands (Southern Adriatic) Italy. Hidrobiologia, pp:75-79.
Ribera, M.A., Gomez Garreta, A., Gallardo, T., Cormaci, M., Furnari, G., Giaccone, G. (1992). Check-list of mediterranean seaweeds. I. Fucophyceae (Warming, 1884). Botanica Marina, 35:109-130.
Delmail, D. (2011). Contribution of Myriophyllum alterniflorum and its periphyton to the biomonitoring of water quality in relation to heavy metals. PhD Thesis in Pharmacology. University of Limoges, France.
Diez, I., Secilla, A., Santolaria, A., Gorostiaga, J.M. (1999). Phytobenthic intertidal community structure along an environmental pollution gradient. Marine Pollution Bulletin, 38:463-472.
Fairweather, P.G. (1990). Sewage and the biota on seashores: assessment of impact in relation to natural variability. Environmental Monitoring and Assessment, 14:197-210.
Falquet, J., Hurni, J.P. (2006). Spiruline: Aspect nutritionnel de la spiruline. Antenna technologies. Genève, p:40.
Francour, P. (1990). Dynamique de l'écosystème à Posidonia oceanica dans le parc national de Port-Cros: analyse des compartiments matte, litière, faune vagile, échinodermes et poissons. PhD Thesis in Biology and Oceanology. University of Paris VI.
Giaccone, G. (1978). Revisione della flora marina del mare Adriatico. Parco Mar. Miramar, 6:1-118.
Gibson, G.R., Bowman, M.L., Gerritsen, J., Snyder, B.D. (2000). Estuarine and coastal marine waters: bioassessment and biocriteria technical guidance. U.S. Environmental Protection Agency, Office of Water, Washington, DC, EPA.
Golubic, S., Brent, G., Lecampion, T. (1970). Scanning electron microscopy of endolithic algae and fungi using a multipurpose casting-embedding technique. Lethaia, 3:203-209.
Haury, J., Dutartre, A., Peltre, M.C. (2008). Les lichens, bryophytes, ptéridophytes et phanérogames aquatiques. Ingénieries, pp:3-5.
Hoffmann, A.A., Parson, P.A. (1988). The analysis of quantitative variation in natural populations with isofemale strains. Génétique, Selection, Evolution, 20:87-98.
Jégou, A. (2011). Territories, stakeholders and stakes in urban sustainability: The case of the Parisian metropolis. PhD thesis in geography. The University of Paris.
Kazzaz, M. (1989). Contribution à l’étude de la flore algale de la région Ouest de la Méditerranée. PhD Thesis. Faculty of Scince of Rabat.
Klein, J. (2007). Impact de Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) sur les communautés macrophytiques en Méditerranée nord-occidentale. PhD Thesis. University of Aix-Marseille II.
Klein, J., Verlaque, M. (2008). The Caulerpa racemosa invasion: A critical review. Marine Pollution Bulletin, 56:205-225.
Lamouti, S., Rebzani, C., Bachari, N.E.I. (2011). Distribution of two introduced species with invasive character in the central region of the Algerian coast: Caulerpa racemosa and Oculina patagonica. Proceedings of the "Mediterranean Coastal and Maritime Conference" (Tanger), pp:361-366.
Mammeria, A.B. (2006). Eutrophisation en Méditerranée: Etat actuel de l’herbier de posidonie Posidonia oceanica dans le golf d’Annaba. Bulletin de l’INSTM: Actes des 8èmes Journées des Sciences de la Mer, 11:24-28.
Marfaing, H., Lerat, Y. (2007). "Do algae have a place in nutrition?" Phytotherapy, 5:2-5.
Murray, S.N., Littler, M.M. (1978). Patterns of algal succession in a perturbated marine intertidal community. Journal of Phycology, 14:506-512.
Orfanidis, S., Panayotidis, P., Ugland, K.I. (2011). Ecological Evaluation Index continuous formula (EEI-c) application: a step forward for functional groups, the formula and reference condition values. Mediterranean Marine Science, 2:199-232.
Ould Ahmed, N. (1994). Study of phytobenthic species in the vicinity of the thermal power plant of Mers El Hadjdj (Gulf of Arzew; West Algeria). Special mention on a remarkable species Chlorophyte, Caulerpales: Caulerpa prolifera (Forsskal) Lamouroux. PhD Thesis. Institute of Marine Sciences and Coastal Planning (ISMAL), Algiers.
Ould Ahmed, N., Meinesz, A. (2007). First record of the invasive alga Caulerpa racemosa (Caulerpales, Chlorophyta) on the coast of Algeria. Cryptogamie, Algologie, 3:303-305.
Ould Ahmed, N., Gómez Garreta, A., Ribera Siguan, M.A., Bouguedoura, N. (2013). Checklist of the benthic marine macroalgae from Algeria. Anales del Jardín Botánico de Madrid, 70:136-143.
Perret-Boudouresque, M., Seridi, H. (1989). Inventaire des algues marines benthiques d'Algérie. GIS Posidonie publ., Marseille, pp:1-117.
Piazzi, L, Ceccherelli, G. (2006). Persistence of biological invasion effects: Recovery of macroalgal assemblages after removal of Caulerpa racemosa var. cylindracea. Estuarine Coastal and Shelf Science, 68:455-461.
Riadi, H. (2000). Etude de la flore marine benthique (Chlorophyceae et Phaeophyceae) du Détroit et de la Méditerranée Occidentale Marocaine (Floristique, Chorologie, Synécologie et Biogéographie). PhD Thesis in Sciences. Universite of Abdelmalek Essaadi, Facultyof Sciences, Tétouan.
Rodriguez-Prieto, C., Polo-Alberti, L. (1996). Effects of the sewage pollution in the structure and dynamics of the community of Cystoseira mediterranea (Fucales, Phaeophyceae). Scientia Marina, 60:253-263.
Scammacca, B., Giaccone, G., Pizzuto, F., Alongi, G. (1993). La vegetazione marina di substrato duro dell'isola di Lampedusa (Isole Pelagie). Bullettin of the Gioenia Academy of Natural Sciences of Catania, 25:115-144.
Seridi, H., Kabrane, K. (2010). Progression of Caulerpa racemosa (Caulerpales, Chlorophyta) on the Algerian coast. Proceedings of the "4th Mediterranean Symposium on Marine Vegetation", Édit. RAC/SPA (Regional Activity Centre for Specially Protected Areas), Tunis, pp:125-128.
Seridi, H. (1990). Etude des algues marines benthiques de la région d’Alger. Thèse de Magister. ISN. USTHB.
Seridi, H. (2004). Algues macrophytes de la côte algérienne. In Biodiversité marine et côtière algérienne. Sonatrach and Grimes (Ed.), pp:79-93.
Seridi, H. (2007). Study of the algal flora of Algeria, phytosociological study of the photophilic algal populations of the superficial infralittoral of hard substratum. PhD Thesis in Sciences. University of Science and Technology Houari Boumediene (USTHB), Algiers.
Seridi, H., Ruitton, S., Boudouresque, C.F. (2007). Is it possible to calibrate the pollution level of the region of Algiers (Mediterranean Sea) by exploiting marine macrophytes? Comptes Rendus Biologies, 330:606-614.
Shannon, C.E. (1948). A mathematical theory of communication. The Bell System Technical Journal, 27:379-423.
Soto, J., Conde, F. (1993). Datos sobre la flora bentonica de la islade Alboran (Mar de Alboran, Méditerranéo occidenta). Criptogamie Algologie, 14:183-190.
Verlaque, M., Boudouresque, C.F., Meinesz, A., Giraud, G., Marcot-Coqueugniot, J. (1977). Marine vegetation of Corsica (Mediterranean) II. Documents for the flora of algae. Vie et Milieu, 27:437-456.
Verlaque, M., Seridi, H. (1991). Antithamnion algeriensis nov. sp. (Ceramiaceae, Rhodophyta) from Algeria (Mediterranean Sea). Botanica Marina, 34:153-160.
Verlaque, M., Fritayre, P. (1994). Impact of the introduced alga Caulerpa taxifolia on the phytobenthos of the western Mediterranean. Photophilic algal communities of the infralittoral. First International Workshop on Caulerpa taxifolia, Boudouresque C.F, Meinesz A., Gravez. (Eds.), GIS Posidonie Publication. Marseille, pp:349-353.
Author Info
S. Mehiaoui1,2*, F. Nemchi2, Z. Bouzaza1, T. Farah3 and B. Bachir-Bouiadjra42Laboratory of Structure, Elaboration and Application of Molecular Materials (SEAMM), Faculty of Natu, Abedelhamid Ibn Badis University of Mostaganem, Algeria
3Laboratory of Biodiversity and Conservation of Water and Soil (LBCWS), Faculty of Natural and Life S, University Abdelhamid Ibn Badis of Mostaganem, Algeria
4Laboratory of Science and Technology of Animal Production (LSTPA), Faculty of Natural and Life Scien, Abedelhamid Ibn Badis University of Mostaganem, Algeria
Citation: Mehiaoui, S., Nemchi, F., Bouzaza, Z., Farah, T., Bachir-Bouiadjra, B. (2022). Algal diversity study in the western Algerian coast. Ukrainian Journal of Ecology. 12:1-11.
Received: 15-Apr-2022, Manuscript No. UJE-22-60870; , Pre QC No. P-60870; Editor assigned: 18-Apr-2022, Pre QC No. P-60870; Reviewed: 02-May-2022, QC No. Q-60870; Revised: 07-May-2022, Manuscript No. R-60870; Published: 14-May-2022, DOI: 10.15421/2022_368
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.