Research - (2022) Volume 12, Issue 4
Adaptation strategies of Moringa oleifera under drought and salinity stresses
S. Bekka1*, K. Tayeb-Hammani1, I. Boucekkine1, M.Y. El-Amin Aissiou1 and Z.E. Djazouli1,2Abstract
The search for a better adaptation to environmental variation has become prominent to yield stabilization. Indeed, the key points in the environmental variation are due to the sensitivity of plants to different biotic and abiotic stresses that characterize the environmental production. Moringa oleifera is a medicinal plant recognized for its wide use in the fields of agronomy, phytotherapy and nutrition. To achieve that, the objective of this study is to investigate the effect of abiotic stress on the physiological responses of M. oleifera plants subjected to different types of stresses, namely drought stress for 20 days and a different concentrations of NaCl (5 and 10 g/L). Different constraints were applied to test the influence of environmental factors on plant growth, relative water content (RWC), Total non-enzymatic antioxidant capacity (TAC), photosynthetic pigment content, endogenous free proline and phenolic compounds. Plants subjected to different stress conditions were compared with control. However, there is a considerable variation among the plants studied in response to different types of abiotic stresses. In this study, we found that abiotic stress induces a series of morphological disturbances in M. oleifera plants, resulting in reduced growth and RWC. Also there was presence of significant reduction in carotenoid levels. However, the adaptive responses of M. oleifera to various stresses have been observed by a strong accumulation of endogenous proline and an increase in total polyphenol content and radical scavenging activity (RAS). These results could be useful for evaluation of the degree of adaptation of plant growth under environmental constraints.
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
Keywords
Moringa oleifera, Abiotic stress, Antioxidant activities, Radical scavenging activity.
Introduction
Environmental constraints are the main limiting factors of agriculture and crop yield various abiotic stresses are distinguished such as water availability (drought and floods), extreme temperatures (cold, frost, heat), salinity, nutritional deficiencies etc., (Verma et al., 2013). Salinity and drought are the two main factors limiting the growth and development of plants. They exert osmotic stress on plants, disrupting cell structure and physiological function (Larcher, 2003). If one of the stresses becomes too severe and exceeds the limits of the plant's resistance, the stress will use the additional energy to activate new physiological and/or biochemical mechanisms that allow it to adapt to these adverse conditions (Wang et al., 2003). In response to abiotic stresses, plants develop a number of strategies that vary by species and environmental conditions. The plant response to stress is complex, as it depends on the severity of the stress, the duration, the stage of development and the state in which the stressed plant is claimed (Yordanov et al., 2000; Wang and Frei, 2011). Therefore, it is extremely important to know the basic indicators that can characterize the resistance of plants to abiotic stresses. The overall resistance of a plant to stress appears to be the result of numerous phenological, anatomical, morphological, physiological, biochemical and molecular modifications that interact to allow the maintaining growth, development and production (Singhal et al., 2016). A strategy is defined, at the plant level, as an interconnected set of adaptations, subject to natural selection, promoting growth and reproduction in a particular environment (Craine, 2009). Coping with plant stresses and climate change, plants develop several adaptive strategies that vary depending on the species and environmental conditions. Each specie will respond to stress with different resistance strategies for survival (Chaves et al., 2003). Generally, these strategies have been grouped into three categories: escape, avoidance, and tolerance (Levitt, 1980; Kooyers, 2015). Tolerance is one of the strategies that allows the plant to perform its physiological functions despite the deterioration in its water state (Passioura, 1996). Under stressful conditions, plants develop defense mechanisms. Among these mechanisms, osmotic regulation plays an important role in plant stress tolerance or resistance. The plant will have to synthesize organic solutes to regulate its water potential (Morgan, 1984; Farooq et al., 2009). Another stress coping strategy is to activate the antioxidant system. Indeed, oxidative stress is the cause of great number of abiotic stresses, thus plant cells implement several detoxification systems such as substances secondary metabolite, that play an important role in the detoxification process of reactive oxygen species (ROS) (Apel and Hirt, 2004; Wang and Frei, 2011). Due to its special environmental, medicinal and dietary properties, Moringa oleifera is a versatile plant whose leaves, flowers, fruits, bark and roots can be consumed directly (Islam et al., 2021). This planthas been widely naturalized in tropical and subtropical regions of the world. Its cultivation has been reported from various parts of Africa, Arabia, Southeast Asia and South America. M. oleifera has gained importance due to its multipurpose uses and good adaptability to both humid and dry climates. These features facilitated its naturalization process in such diverse regions (Gedefaw, 2015; Gandji et al., 2018). Accordingly, the purpose of this study is to investigate the adaptive responses of M. oleifera subjected to water and salt stresses by measuring antioxidant capacity, radical scavenging activity and endogenous free proline under normal and stressful conditions.
Materials and Methods
Plant material
Moringa oleifera seeds were purchased from a local market and were originally imported from India. This plant was selected due to its usefulness and bioactive contents (phenolic compounds).
Cultivation
Seeds germinated 3 days after transplanting them into a pot containing 1/3 sand and 2/3 potting soil for easy drainage and regular watering. Growth will take place for 3 months in a culture chamber, exposed to a cycle of 16 h light and 8 h darkness at a temperature of 30°C.
Application of the different treatments
Water and salt stresses are applied in the 3rd month of growth.
Drought stress: Was induced by stopping irrigation for about 20 days.
Salinity: Caused by sodium chloride (NaCl) in increasing concentrations of 5 and 10 g/L.
Depending on the treatment, the plants are watered with 250 mL of water/solution.
Control (C): Irrigation water
Stressed 1(S1): Not watered for 20 days
Stressed 2(S2): Irrigated with 5% NaCl
Stressed 3(S3): Irrigated with 10% NaCl
Growth parameters
• Measurements of the length of the aerial parts of the control and stressed plants (expressed in cm), are made using a graduated ruler at the 3rd month of growth.
• The aerial parts of each plant were weighed separately in the fresh state (Fresh mass, FM), then in the dry state (dry mass, DM), after going in oven for 48 h at 80°C.
Determination of relative water content (RWC)
Leaves’ turgor was evaluated by the determination of RWC according to Barrs (1968). The leafs discs mass was evaluated straight away after sampling the fresh mass and incubating the sample in distilled water for 24 h (mass at full turgor). Afterwards, they were put in the oven and dried for 24 h at 80°C until their dry mass was obtained. Finally, we calculated the relative water content using the following formula:
RWC=(fresh mass-dry mass) × 100/(fresh mass of full turgor-dry mass)
Determination of photosynthetic pigments content
The chlorophyll a, band total carotenoids contents were measured according to Lichtenthaler (1987). A sample of 15 mg of Moringa leafs was ground in 1.5 mL of 80% acetone.
The calculation of the chlorophyll a, chlorophyll band carotenoid content were based on the following formulae:
Chl a (μg mL-1) = 12.25 × OD663-2.79 × OD647
Chl b (μg mL-1) = 21.5 × OD647-5.10 × OD663
Chl a+Chl b (μg mL-1) = 7.15 × OD663+18.71 × OD647
Total carotenoids (μg mL-1) = (1000 * D.O470-1.82) × (Chl a-85.02)/198
Free proline content
The amount of proline in the leaves was carried out according to the approach adopted by Troll and Lindsley (1955) and modified by Magné and Larher (1992). A Sample of 50 mg dry mass of leaves was homogenized with 1 mL of distilled water at 90°C for the duration of 30 min in a water bath. After centrifugation at 12000 rpm for 10 min, we added 500 μL aliquot of the supernatant with 1 mL of the reagent mixture (1 g ninhydrin, 40 mL distilled water and 60 mL glacial acetic acid). Afterwards, the mixture was heated at 95°C for 30 min in sealed tubes. After cooling down the samples, 3 mL of toluene was added. The content of proline was determined by a spectrophotometer at 520 nm and expressed as mg.g-1 DM.
Determination of phenolic compounds
An amount of 1 g of ground sample was added with 50 mL of 80% methanol. The mixture was left for agitation during 24 h in darkness. After centrifugation at 6000 rpm for 15 min, the supernatant was used to estimate the phenolic compounds content, radical scavenging activity (RSA) and total non-enzymatic antioxidant capacity (TAC).
Total phenol content (TPC)
The assessment of TPC was carried out by using the protocol of Singleton et al., (1999). A volume of 200 µL of supernatant was put in 1.5 mL of the Folin-Ciocalteu reagent (diluted 10 times), and added by 1.5 mL of sodium carbonate solution. After vortexing the mixture for homogenization, it was left for incubation in the dark for 90 min. The absorbance of samples was measured by spectrophotometry at 725 nm. The phenolics quantification was based on the standard curve of a gallic acid and expressed as mg gallic acid equivalent (GAE) per g of DM.
Total flavonoid content
The flavonoids content was evaluated based on the Aluminium chloride method described by Lamaison and Carnat, (1990). 1 mL of methanolic extract was mixed with 2% of aluminium chloride solution (AlCl3) dissolved in methanol. The mixture was incubated at room temperature for 15 min. The absorbance was determined by spectrophotometry at 430 nm. The flavonoid quantification was based on the standard curve of quercetin and expressed in mg quercetin equivalent (QE) per g of DM.
Total non-enzymatic antioxidant capacity (TAC)
Total non-enzymatic antioxidant capacity in Moringa leaves was estimated by the method of Prieto et al., (1999). 200 μL of each extract was mixed with 1 mL of ammonium molybdate reagent (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were incubated in a water bath at 95°C for 90 min. After cooling the mixture to room temperature; the absorbance of the solution was measured at 695 nm. TAC is expressed in mg ascorbic acid equivalent (AAE) per g of DM.
Measurement of radical-scavenging activity (RAS) by the DPPH test
The efficiency of the extracts to scavenge the DPPH free radical was evaluated according to the method described by Afify et al., (2012). The DPPH radical is dissolved in methanol at a concentration of 5 mg/150 mL. To each extract (100 μL) is added 2900 μL of DPPH solution. The mixture is incubated in darkness at room temperature for 30 min. The absorbance was measured at 515 nm. The RSA of DPPH was determined with the following formula:
RSA%=100 × (absorbance of control-absorbance of sample/absorbance of control)
Statistical analysis
Experiments have been carried out at least five times. Means (M) and standard errors (SE) were given as (M ± ES). The statistical analyses, based on Student's test, were performed using MS-EXCEL-2007 (Microsoft Corp) software. The results showing a value of p ≤ 0.05 were considered as statistically significant.
Results
Shoot height and biomass of leaves
The effects of water and salt stress on growth were evaluated after 3 months of growth, by measuring the shoot length of the aerial part as well as the weight of the fresh and dry mass of the leaves. Based on the results depicted in Table 1, water and salt stress have significantly (p ≤ 0.05) reduced the aerial plant growth compared to the control. Thus, reduction for stressed (S1) was 30% whereas the expressed reductions for both stressed S2 and S3 reached respectively 16.58% and 31.79%.
| Treatments | Shoot Height (cm) | FM of Leaves (mg) | DM of Leaves (mg) |
|---|---|---|---|
| C | 54.25 ± 0.37a | 25 ± 0.77a | 6.4 ± 0.08a |
| S1 | 37.75 ± 0.18b | 15 ± 1.85b | 3.3 ± 0.81b |
| S2 | 46.25 ± 0.17b | 17.33 ± 1.41b | 4.43 ± 0.45b |
| S3 | 37.00 ± 0.25b | 20.67 ± 1.03b | 5.71 ± 0.46a |
Table 1. Effect of abiotic stress on height and shoot biomass of Moringa oleifera leaves. C: Control; S1: Drought stress; S2:5% of NaCl; S3:10% of NaCl.
Along with this growth decline, drought stress also induced a sharp reduction in the weight of the fresh and dry mass of the leaves 40% and 48.43% respectively (Table 1). The decreases in the fresh and dry weight of M. oleifera ‘s leaves are more important in plants watered by 5% of NaCl 30.68% for fresh mass and 30.78% for dry mass. While this reduction is estimated at 17.32% (fresh mass) and 10.78% (dry mass) respectively in plants watered at 10% of NaCl.
Effect of drought and salinity stresses on relative water content (RWC) of Moringa oleifera’s leaves
The relative water content is considered as an excellent indicator of the water status of the plant. Under the conditions of our experiment, the analysis of the results obtained in Fig. 1 demonstrated that, in case of a water stress situation, the RWC declines by 17.49% compared to the control. The presence of 5% NaCl in the medium caused a reduction of 8.95% in RWC. This reduction is greater in the plant watered by NaCl at 10% (25.96%) compared to the control.
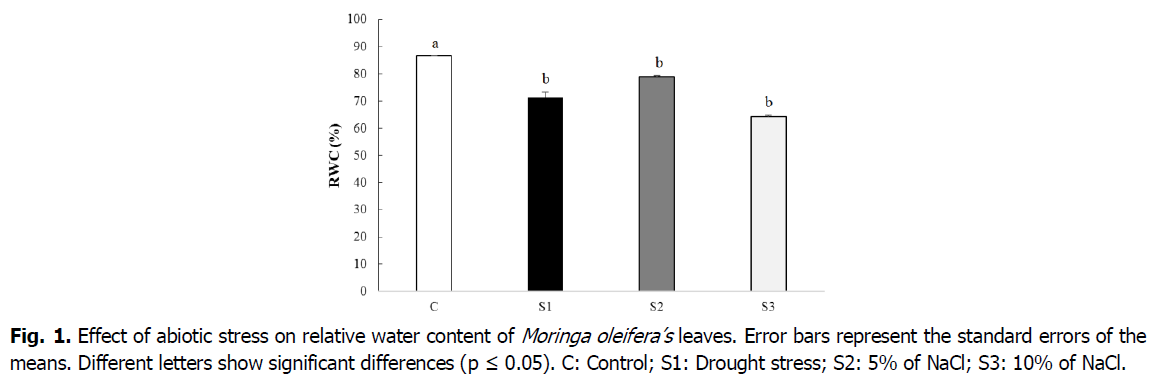
Fig 1. Effect of abiotic stress on relative water content of Moringa oleifera’s leaves. Error bars represent the standard errors of the means. Different letters show significant differences (p ≤ 0.05). C: Control; S1: Drought stress; S2: 5% of NaCl; S3: 10% of NaCl.
Chlorophyll content
From Table 2, the chlorophyll a and b contents of M. oleifera’s leaves is not significantly affected by water stress (p ≤ 0.05). Similarly, the saline concentrations tested had no negative effect on chlorophyll a and b contents. Nevertheless, under drought and saliny conditions, the total carotenoids contents were significantly (p ≤ 0.05) decreased. Thus, we noted a reduction rate of 70% for stressed (S1); 57.50% and 40% for stressed (S2; S3) respectively compared to the control.
| Treatments | Chlorophyll a (mg.g-1 FM) | Chlorophyll b (mg.g-1 FM) | Total carotenoids (mg.g-1 FM) |
|---|---|---|---|
| C | 1.53 ± 0.18a | 2.23 ± 0.10a | 0.40 ± 0.03a |
| S1 | 1.26 ± 0.22a | 2.20 ± 0.07a | 0.12 ± 0.01b |
| S2 | 1.49 ± 0.13a | 2.17 ± 0.14a | 0.17 ± 0.04b |
| S3 | 1.62 ± 0.34a | 1.95 ± 0.11a | 0.24 ± 0.03b |
Table 2. Effect of abiotic stress on chlorophylls and carotenoids contents of Moringa oleifera’s leaves. C: Control; S1: Drought stress; S2: 5% of NaCl; S3:10% of NaCl.
Effect of drought and salinity stress on endogenous free proline content of Moringa oleifera’s leaves
The accumulation of foliar free proline increased significantly (p ≤ 0.05)under the effect of water stress in stressed (S1) (0.091 mg.g-1 DM) as the triple of the control (0.034 mg.g-1 DM). This corresponds to an increase rate of 167.64% (Fig. 2). Free proline content increased significantly (p ≤ 0.05) with salt concentration increase (Fig. 2). Plants watered at 5% and 10% of NaCl had increased rates respectively of 174.47% and 194.11% compared to the control.
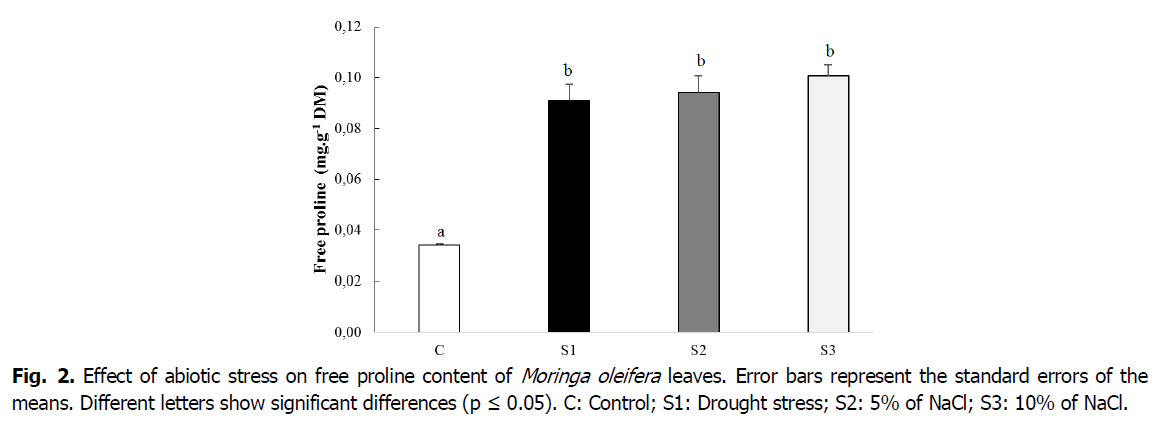
Fig 2. Effect of abiotic stress on free proline content of Moringa oleifera leaves. Error bars represent the standard errors of the means. Different letters show significant differences (p ≤ 0.05). C: Control; S1: Drought stress; S2: 5% of NaCl; S3: 10% of NaCl.
Effect of drought and salinity stress on phenolic compounds and TAC of Moringa oleifera’s leaves (GAE) per g of DM
Phenolic compounds
The water stress as a whole induced a significant increase (p ≤ 0.05) in the content of phenolic compounds in the leaves of stressed (S1), thus leading to an increase in polyphenols (35.05%) and (10. 84%) in flavonoids compared to the control (Table 3). Also, an increase of 66.04% for phenols and 86.47% for flavonoids was recorded in plants watered with saline solution at 5%. Nevertheless, this increase is less in stressed (S3) estimated at 40.33% for polyphenols and 34.93% for flavonoids.
| Treatments | PT mg (GAE).g-1 of DM | FT mg (QE).g-1 of DM | TAC mg (AAE).g-1 of DM |
|---|---|---|---|
| C | 67.01 ± 0.43b | 0.80 ± 0.01b | 1.03 ± 0.01c |
| S1 | 90.49 ± 1.16a | 0.92 ± 0.02a | 2.15 ± 0.30b |
| S2 | 111.27 ± 2.77a | 1.55 ± 0.10a | 2.41 ± 0.09b |
| S3 | 94.04 ± 2.43a | 1.12 ± 0.03a | 3.43 ± 0.24a |
Table 3. Effect of abiotic stress on phenolic compounds and TAC of Moringa oleifera leaves. C:Control; S1:Drought stress; S2: 5% of NaCl; S3: 10% of NaCl.
Total non-enzymatic antioxidant capacity (TAC)
Water stress caused a significant (p ≤ 0.05)increase in TAC in M. oleifera’s leaves, resulting in an increase rate of 108.73% compared to control, (Table 3). The effect of salinity on antioxidant capacity depends on the applied concentration. Indeed, we noticed that the TAC is inferior in S2 than S3. More precisely, an increase rate of 133.98% was recorded in the stressed (S2), while, it is estimated at 233% in stressed (S3) compared to the control (Table 3).
Effect of drought and salinity stress on DPPH radical scavenging activity of Moringa oleifera’s leaves
The obtained results showed that water stress led to a significant increase (p ≤ 0.05) in the RAS of stressed (S1). It increased from 60.42% in the control to 92.95% in the stressed (S1); with a rate of 53.83% compared to the control (Fig. 3). Leaf extracts of plants treated with NaCl at 5% showed a better RAS (+35.86%) than that of leaves S3 (+5.95%) compared to control (Fig. 3).
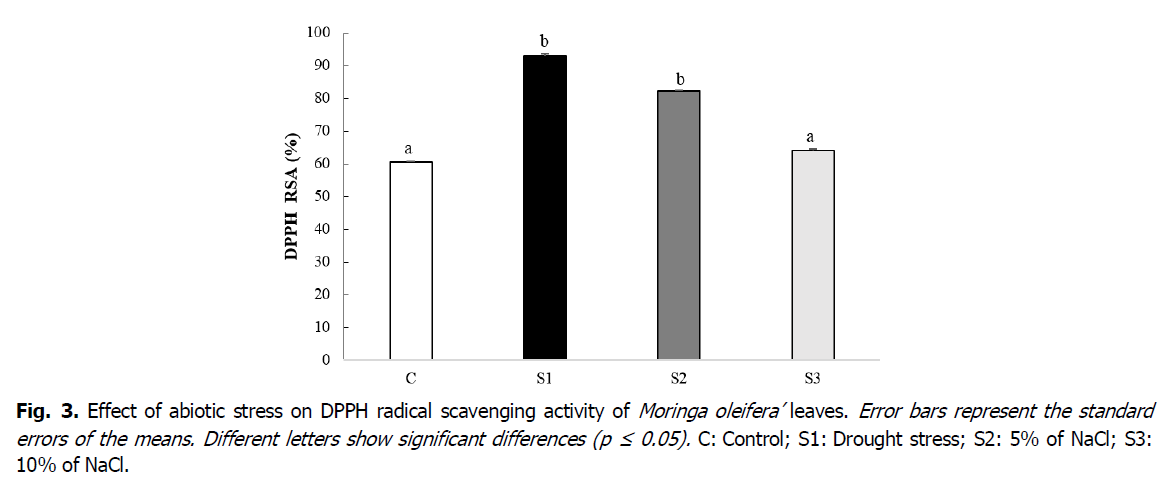
Fig 3. Effect of abiotic stress on DPPH radical scavenging activity of Moringa oleifera’ leaves. Error bars represent the standard errors of the means. Different letters show significant differences (p ≤ 0.05). C: Control; S1: Drought stress; S2: 5% of NaCl; S3: 10% of NaCl.
Discussion
Growth
The results showed that the different stresses applied influence significantly shoot height and biomass of M. oleifera plants. We believe that this decrease is related to the decrease in water potential and turgor of the cell. This results in a decrease in cell enlargement with the consequence of growth inhibition. Our hypothesis is in line with the conclusions of many researchers, including Boumenjel et al., (2021) who evaluated the level of adaptation and tolerance of Moringa to water stress simulated by polyethylene glycol (PEG-6000) (-0.5, -1 and -1.5 MPa). They also have demonstrated that the higher the concentration of PEG-6000, the greater the reduction in growth. Similarly, Hussein and Abou-Baker (2013) assessed the growth response of M. oleifera under saline conditions. These researchers speculated that the detrimental effect of salinity on growth could be the result of a reduction in the water availablity in the root zone causing a water deficit, phototoxicity of Na+ and Cl– ions, and an imbalance depressing nutrient absorption and transport due to excess of Na+. According to Anjum et al., (2011), the decrease in the biomass of Moringa’s leaves under abiotic stress may be due to the reduction of cellular turgor in response to low water availability in the soil (drought or salinity).
The relative water content (RWC)
The relative water content is a key indicator of the degree of cells and tissues’s turgor which is crucial for proper physiological functioning and optimal growth. Our results showed that the cessation of watering and the presence of 5 and 10% NaCl in the medium led to a significant decrease in the RWC of the M. oleifera ‘s leaves. This decrease in RWC in plants that have undergone water and saline stresses (5 and 10% NaCl), could be associated with a strong accumulation of endogenic proline which results in a decrease in osmotic potential and an increase in osmotic adjustment capacity. Again, our hypothesis, is in line with findings of Bajji et al. (2001) and Slama et al., (2005), who demonstrated that the fall in osmotic potential under deficit conditions is due to the increase in the concentration of compatible solutes. Gill et al., (2001); Rama and Nataraja (2009) and Kalina et al., (2016), confirmed that lowering the RWC is induced by various abiotic stresses such as drought, salinity, cold and heat stress.
Photosynthetic pigments
The results concerning the variations in the contents of photosynthetic pigments demonstrated that the chlorophylls a and b are not affected by the stresses applied, thus reflecting a better tolerance of Moringa plants to stress. Similarly, research by Gummuluru and Hobbs (1989) and Sahitya et al., (2018) reported that chlorophyll is a good indicator of the water stress tolerance threshold. The higher this parameter, the more stress tolerant the varieties are. These results lead us to conclude that M. oleifera plants tolerate drought and salinity better. In addition, Cornic et al., (2000) estimated that the survival of plants from water deficit is largely due to the maintenance of the photosynthetic capacity of the leaves. Nevertheless, carotenoids are negatively affected under conditions of water and salt stresses. Similar results were also reported under the same stress conditions in M. oleifera by Boumenjel et al., (2021) and Abou El-Leel et al., (2018). The lycopene; β-carotene and xanthophylls contents have been shown to be highly inhibited by water and heat stress (Munne-Bosch and Alegre, 2000; Dumas et al., 2003).
Free proline content
Proline is not only one of the essential amino acids, but also considered as a non-enzymatic antioxidant involved in the response to different environmental stresses and proposed as a regulatory molecule or signal that can activate multiple physiological and molecular responses (Radjeb et al., 2014). The effect of salt and drought stress resulting in significant accumulations of free proline in comparison to the control. Indeed, the imposed water stress considerably increases the proline content was observed. In addition, the proline content increases with the increase of NaCl concentrations. The causes of proline accumulation is related to M. oleifera's ability to adjust its cellular content under deficit conditions. Moreover, it has been proven that the accumulation of proline in stressed plants constitutes metabolic responses of adaptation to environmental stresses (Ashraf and Foolad, 2007). Our results are in agreement with those reported by Abou El-Leel et al., (2018) and Blanchard-Gros et al., (2021). The authors noted an increase in proline of M. oleifera plants; Solanum chilense and Solanum lycopersicum subjected to different types of abiotic stresses (salinity, drought and heat stress). The main role of proline would be to preserve the turgor of the cell by osmotic adjustment, which avoids an efflux of water from the cell. Proline allows plants to increase osmolarity and protect cellular structures under abiotic stress. Several studies have confirmed that the content of proline in stress-resistant plants is higher than in sensitive plants (Nayyar and Walia, 2003; Gonzalez et al., 2006). This supports the idea that the accumulation of this amino acid may be strongly involved in an adaptation mechanism of plants to environmental stresses. This is supported by Chen et al., (1995) who noted a low presence of this amino acid in sensitive plants. The ability to accumulate proline is correlated with plants' tolerance to abiotic stress. It appears to be the best indicator of stress resistance for certain mechanisms (Hare and Cress, 1997).
Phenolic compounds
Several studies have shown that the accumulation of secondary metabolites, including polyphenols and flavonoids have been associated with water stress, salt; thermal and UV radiation (Achakzai et al., 2009; Hasan et al., 2018). Under water and salt stress conditions, we suggest the presence of a relationship between the different stresses applied and the content of phenolic compounds. This assumes that polyphenols are considered as a form of defense against environmental stresses. Comparable results have also been found in M. oleifera subjected to different types of stress such as water stress (Sakr et al., 2016) and salt stress (Nouman et al., 2012), explaining that one of the causes of accumulation of phenolic compounds would be related to the set up of oxidative stress which is at the origin of several abiotic stresses. Hence, the implementation by plants of several detoxification systems including secondary metabolites. It is widely recognized that abiotic constraints are capable of stimulating the biosynthesis of polyphenols (Apel and Hirt, 2004; Hasan et al., 2018). In addition, it is already well known that polyphenols are considered as a major group of compounds that contribute to the antioxidant activities of plants in front of biotic and abiotic stresses; as scavengers of free radicals due to their hydroxyl groups (Dixon and Paiva, 1995). It has been demonstrated by several studies that the key enzyme phenylalanine involved in the phenylpropanoid biosynthesis pathway is strongly stimulated by various abiotic stresses (Kangasjärvi et al., 1994; Guo et al., 2008; Oh et al., 2009; Frei et al., 2010).
TAC
According to Prieto et al., (1999), TAC corresponds to non-enzymatic antioxidants that can behave as free radical scavengers by direct interventions on prooxidant molecules. Our findings showed that abiotic stress caused a considerable increase in TAC with an apparent effect towards stress. Several studies have shown that there is a positive correlation between antioxidant activity and the content of total phenolic compounds in stressed plants (Nouman et al., 2012). By taking into account, the results of this study and those reported by Nouman et al. (2012), we were able to highlight the existence of a close relationship between the phenolic compounds content (Table 3) of M. oleifera ‘s leaves extracts and their antioxidant capacities.
DPPH radical-scavenging activity
Results of the antioxidant activity of stressed plants towards the DPPH. radical showed higher RAS in stressed (S1) and (S2) compared to that of control. According to Wong et al., (2006) and Chang et al., (2012), radical scavenging helps to sweep away the potential damage caused by free radicals. The increase in RAS in stressed plants S1 and S2 could be explained by a high accumulation of antioxidants such as flavonoids following stimulation of the genes’ expression involved in their biosynthesis pathway in response to stress (Guo et al., 2008; Oh et al., 2009).
Conclusion
Environmental constraints represent major risks to agricultural production. Understanding the mechanisms of tolerance and/or resistance of cultivated plants to different abiotic stresses is a lever for the sustainability of plant production. The results of the present study showed that Moringa oleifera expressed an adaptive ability to stressful conditions throughout the development of certain physiological and biochemical mechanisms; stimulating their non-enzymatic antioxidant system and an increased accumulation of endogenous free proline. Our results also revealed that M. oleifera plants adopted different adaptive strategies related to the type of the applied stress. Moreover, there appears a certain disparity in the amplification of the total antioxidant capacity and the radicalscavenging activity. The adaptive responses of this medicinal plant to different types of abiotic stress allow us to spread its cultivation on soils affected by salinity or water deficit.
References
Abou El-Leel, O.F., El-Shayeb, N.S., El-Azzony, E.A. (2018). Effect of proline on growth and the active ingredients of Moringa oleifera LAM. Plant under salt stress. Journal of Microbiology, 51:56-84.
Achakzai, A.K.K., Achakzai, P., Masood, A., Kayani, S.A., Tareen, R.B. (2009). Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pakistan Journal of Botany, 41:2129-2135.
Afify, A.E.M.M., El-Beltagi, H.S., Abd El-Salam, S.M., Omran, A.A. (2012). Biochemical changes in phenols, flavonoids, tannins, vitamine, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pacific Journal of Tropical Biomedicine, 2:203-209.
Anjum, S.A., Xie, X.Y., Wang, L.C., Saleem, M.F., Man, C., Lei, W. (2011). Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research, 6:2026-2032.
Apel, K., Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55:373-399.
Ashraf., M., Foolad., M.R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59:206-216.
Bajji, M., Lutts, S., Kinet, J.M. (2001). Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Science, 160:669-681.
Barrs, H.D. (1968). Determination of water deficits in plant tissues. In: T.T Kozlowski (Eds), Water deficits and plant growth, New York, NY: Academic Press, pp:235-368.
Ben Rejeb, K., Abdelly, C., Savouré, A. (2014). How reactive oxygen species and proline fac stress together. Plant Physiology and Biochemistry, 80:278-284.
Blanchard-Gros, R., Bigot, S., Martinez, J.P., Lutts, S., Guerriero, G. (2021). Comparison of drought and heat resistance strategies among six populations of solanum chilense and two cultivars of Solanum lycopersicum. Plants, 10:1-22.
Boumenjel, A., Papadopoulos, A., Ammari, Y. (2021). Growth response of Moringa oleifera (Lam) to water stress and to arid bioclimatic conditions. Agroforestry Systems, 95:1-11.
Chang, C.J., Tzeng, T.F., Liou, S.S., Chang, Y.S., Liu, I.M. (2012). Acute and 28-Day subchronic oral toxicity of an ethanol extract of zingiber zerumbet (l.) smith in rodents. Evidence-Based Complementary and Alternative Medicine, 10:2-11.
Chaves, M.M., Maroco, J.P., Pereira, J.S. (2003). Understanding plant responses to drought-from genes to the whole plant. Functional Plant Biology, 30:239-264.
Chen, H., Kuang, D., Wang, J. (1995). Studies on selection and characterisation of a stress tolerant sugarcane cell line. Chinese Journal of Biotechnology, 11:9399.
Cornic, G. (2000). Drought stress inhibits photosynthesis by decreasing stomatal aperture not by affecting ATP synthesis. Trends in Plant Science, 5:187-188.
Craine, J.M. (2009). Resource strategies of wild plants. Princeton: Princeton University Press, p:352.
Dixon, R.A., Paiva, N.L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell, 7:1085-1109.
Dumas, Y., Dadomo, M., Di Lucca, G., Grolier, P. (2003). Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. Journal of the Science of Food and Agriculture, 83:369-382.
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., Basra, S.M.A. (2009). Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development, 29:185-212.
Gandji, K., Chadare, F., Idohou, R., Salako, V., Assogbadjo, A., Kakaï, R.G. (2018). Status and utilisation of Moringa oleifera Lam: A review. African Crop Science Journal, 26:137-156.
Gedefaw, M. (2015). Environmental and medicinal value analysis of moringa (Moringa oleifera) tree species in Sanja, North Gondar, Ethiopia. American International Journal of Contemporary Scientific Research, 2:1-17.
Gill, P.K., Sharma, A.D., Singh, P., Bhullar, S.S. (2001). Effect of various abiotic stresses on the growth, soluble sugars and water relations of Sorghum Seedlings grown in light and darkness. Bulgarian Journal of Plant Physiology, 27:72-84.
Gonzalez, H., Martínez-Lozano, S., Nunez-Gonzalez, M.A., Garcia Diaz, G., Hernandez-Pinero, J.L., Morales-Vallarta, M.R. (2006). Variability in accumulation of free proline on in vitro calli of four bean (Phaseolus vulgaris L.) cultivars exposed to salinity and induced moisture stress. Phyton, 75:103-108.
Gummuluru, S., Hobbs, S.L.A. (1989). Genotype variability in physiological characters and its relationship to drought tolerance in durum wheat. Canadian Journal of Plant Science, 69:703-711.
Guo, J., Han, W., Wang, M.H. (2008). Ultraviolet and environmental stresses involved in the induction and regulation of anthocyanin biosynthesis: a review. African Journal of Biotechnology, 7:4966-4972.
Hare, P.D., Cress, W.A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regulation, 21:79-102.
Hasan, M., Alharby, H., Hajar, A., Hakeem, K., Alzahrani, Y., Arabia, S. (2018). Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. The Journal of Animal and Plant Sciences, 28:803-810.
Hayat, S., Hayat, Q., Alyemeni, M.N., Wani, A.S., Pichtel, J., Ahmad, A. (2012). Role of proline under changing environment. Plant Signaling and Behavior, 7:1456-1466.
Hussein, M., Abou-Baker, N.H. (2013). Growth and mineral status of Moringa plants as affected by silicate and salicylic acid under salt stress. International Journal of Plant and Soil Science, pp:163-177.
Islam, Z., Islam, S.M.R., Hossen, F., Mahtab-Ul-Islam, K., Hasan, Md.R., Karim, R. (2021). Moringa oleifera is a prominent source of nutrients with potential health benefits. International Journal of Food Science, 2:1-11.
Kangasjärvi, J., Talvinen, J., Utriainen, M., Karjalainen, R. (1994). Plant defense systems induced by ozone. Plant, Cell and Environment, 17:783-794.
Kooyers, N.J. (2015). The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science, 234:155-62.
Lamaison, J.L.C., Carnet, A. (1990). Teneurs en principaux flavonoides des fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Poiret D.C) en Fonction de la végétation. Pharmaceutica Acta Helvetia, 65:315-320.
Larcher, W. (2003). Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, p:513.
Levitt, J. (1980). Responses of plants to environmental stresses. Water, radiation, salt and other stresses. Academic Presse, New York, p:607.
Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methodes in Enzymology, 148:350-382.
Magné, C., Larher, F. (1992). High sugar content of extracts interfers with colorimetric determination of amino Acids and free proline. Analytical Biochemistry, 200:115-118.
Morgan, J.M. (1984). Osmoregulation and water stress in higher plants. Annual Review Plant Physiology, 35:299-319.
Munne-Bosch, S., Alegre, L. (2000). Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta, 210:925-931.
Nouman, W., Siddiqui, M.T., Basra, S.M.A., Khan, R.A., Gull, T., Olson, M.E., Hassan, M. (2012). Response of Moringa oleifera to saline conditions. International Journal of Agriculture and Biology, 14:757-762.
Oh, M.M., Trick, H.N., Rajashekara, C.B. (2009). Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. Journal of Plant Physiology, 166:180-191.
Passioura, J.B. (1996). Drought and drought tolerance. Plant Growth Regulation, 20:79-83.
Prieto, P., Pineda, M., Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry, 269:337-341.
Rama, N., Nataraja, K.N. (2009). Down-regulation of an abiotic stress related Nicotiana benthamiana WRKY transcription factor induces physiological abnormalities. Indian Journal of Biotechnology, 8:53-60.
Sahitya, U.L., Krishna, M.S.R., Sri Deepthi, R., Prasad, G.S., Kasim, D.P. (2018). Seed antioxidants interplay with drought stress tolerance indices in chilli (Capsicum annuum L) seedlings. BioMed Research International, pp:1-15.
Sakr, M., Darwish, M., Ibrahim, H., Mostafa, N. (2016). Physiological effects of some antioxidants on Moringa (Moringa oleifera, L.) plant under drought stress. Journal of Plant Production Mansoura University, 7:351-360.
Singhal, P., Jan, A.T., Azam, M., Haq, Q.M.R. (2016). Plant abiotic stress: a prospective strategy of exploiting promoters as alternative to overcome the escalating burden. Frontiers in Life Science, 9:52-63.
Singleton, V.L., Orthofer, R., Lamuela-Raventós, R.M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299:152-178.
Soltys-Kalina, D., Plich, J., Strzelczyk-Żyta, D., Śliwka, J., Marczewski, W. (2016). The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding Science, 66:328-331.
Slama, A., Ben Salem, M., Ben Naceur, M., Zid, E. (2005). Les céréales en Tunisie: production, effet de la sécheresse et mécanismes de résistance. Sècheresse, 16:225-229.
Troll, W., Lindsey, J. (1955). A photometric method for the determination of proline. Journal of Biological Chemistry, 215:655-660.
Verma, S., Nizam, S., Verma, P.K. (2013). Biotic and abiotic stress signaling in plants. In: Sarwat, M., Ahmad, A. and Abdin, M., (Eds.), Stress signaling in plants: genomics and proteomics perspective. Springer Science, New York, 1:25-49.
Wang, W., Vinocur, B., Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta, 218:1-14.
Wang, Y., Frei, M. (2011). Stressed food-The impact of abiotic environmental stresses on crop quality. Agriculture, Ecosystems and Environment, 141:271-286.
Wong, S.P., Leong, L.P., William, J.H. (2006). Antioxidant activities of aqueous extracts of selected plants. Food Chemistry, 99:775-783.
Yordanov, I., Velikova, V., Tsonev, T. (2000). Plant responses to drought, acclimation, and stress tolerance. Photosynthetica, 38:171-186.
Author Info
S. Bekka1*, K. Tayeb-Hammani1, I. Boucekkine1, M.Y. El-Amin Aissiou1 and Z.E. Djazouli1,22Department of Biotechnology, Faculty of Natural and Life Sciences, Laboratory of Biotechnology of Plant Productions, University of Blida, 1, B.P. 270, Route De Soumaa, Blida, Algeria
Citation: Bekka, S., Tayeb-Hammani, K., Boucekkine, I., El-Amin Aissiou, M.Y., Djazouli, Z.E. (2022). Adaptation strategies of Moringa oleifera under drought and salinity stresses. Ukrainian Journal of Ecology. 12:8-16.
Received: 25-Feb-2022, Manuscript No. UJE-22-55578; , Pre QC No. P-55578; Editor assigned: 28-Feb-2022, Pre QC No. P-55578; Reviewed: 12-Mar-2022, QC No. Q-55578; Revised: 18-Mar-2022, Manuscript No. R-55578; Published: 30-Mar-2022, DOI: 10.15421/2022_359
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
